A new, multidisciplinary center aims to accelerate and streamline the translation of potential therapeutic targets into novel treatment options.
Clinical Collaboration, Surgical Advances Boost Lung Cancer Prevention and Treatment
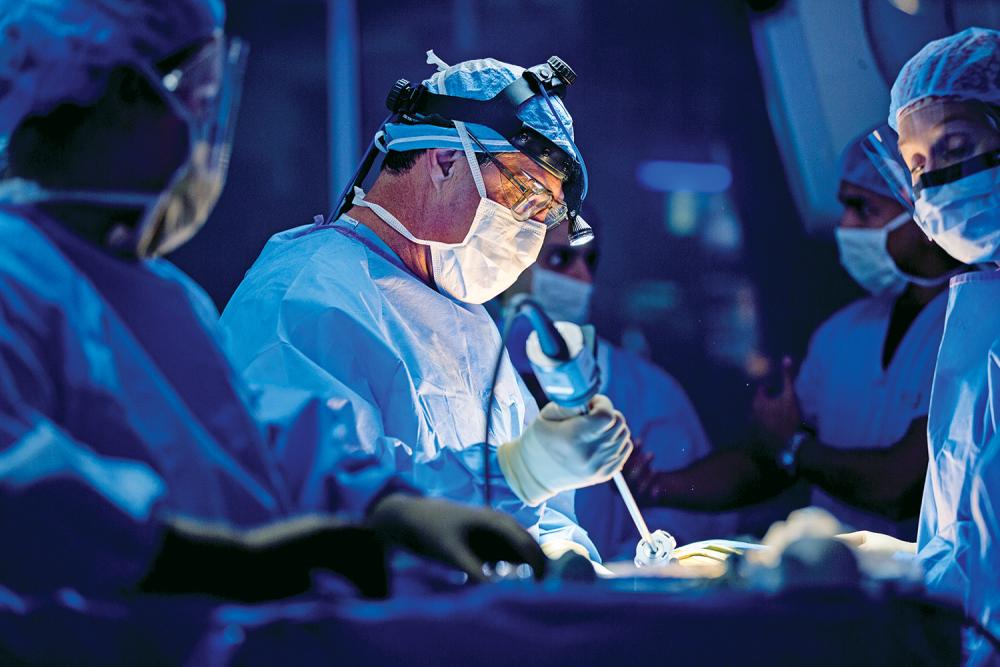
Robot-assisted surgery, telemedicine, and the latest in genomic research anchor Perlmutter Cancer Center’s new multidisciplinary Lung Cancer Center. The center brings together new and existing programs to study, diagnose, treat, and ultimately prevent the nation’s leading cause of cancer-related deaths.
Robert J. Cerfolio, MD, MBA, who has performed more robotic thoracic procedures than any surgeon worldwide and developed innovations that have given rise to global changes in lung cancer surgery, joined Perlmutter Cancer Center in 2017 to lead the team.
The mission of the Lung Cancer Center is two-fold: to bring new discoveries to the clinical setting more rapidly and efficiently, and to improve clinical collaboration and community outreach. Dr. Cerfolio, professor of cardiothoracic surgery and director of clinical thoracic surgery, has trained many practicing thoracic surgeons and will continue this emphasis at the new center.
In parallel with promoting surgical advances, the multidisciplinary nature of the center will strengthen researchers’ existing inquiries into disease progression and novel therapies, such as the immunotherapy studies underway by Leena Gandhi, MD, PhD, associate professor of medicine and director of thoracic medical oncology.
In addition to Dr. Cerfolio and Dr. Gandhi, the new center includes, among others:
- Abraham Chachoua, MD, the Jay and Isabel Fine Professor of Oncology, professor of urology, and associate director for cancer services
- Harvey I. Pass, MD, the Stephen E. Banner Professor of Thoracic Oncology, professor of cardiothoracic surgery, director of thoracic oncology, and director of the Division of Thoracic Surgery
- Peter B. Schiff, MD, PhD, professor of radiation oncology, director of faculty affairs, and vice chair of the Department of Radiation Oncology
- Benjamin Cooper, MD, assistant professor of radiation oncology
- Daniel H. Sterman, MD, the Thomas and Suzanne Murphy Professor of Pulmonary and Critical Care Medicine, professor of cardiothoracic surgery, director of the Multidisciplinary Pulmonary Oncology Program, and director of the Division of Pulmonary, Critical Care, and Sleep Medicine
- Kwok-Kin Wong, MD, PhD, the Anne Murnick Cogan and David H. Cogan Professor of Oncology and director of the Division of Hematology and Medical Oncology
Robert J. Cerfolio, MD, MBA, director of the Lung Cancer Center, has performed more robotic thoracic surgeries than any other surgeon in the world.
Genetic Research Identifies Growing Pains, New Therapeutic Vulnerability in Lung Cancer Cells
Genetic changes that help non-small cell lung cancer thrive also make it vulnerable to a promising experimental drug, according to a study led by Thales Y. Papagiannakopoulos, PhD, assistant professor of pathology. The research was published online in October 2017 in Nature Medicine.
Using the CRISPR-Cas9 gene editing system to make changes in KEAP1 similar to those in about 10 percent of lung cancers, researchers found that weakening the gene’s function increased the production of antioxidants that help cancer cells thrive despite oxidative stress—a damaging by-product of their KRAS-mutation–driven, energy-intensive growth. Because lung cancer cells with co-occurring mutations in KEAP1 and KRAS must use large amounts of glutamate to keep oxidative stress at bay, they become highly dependent on this amino acid.
Recognizing this “competition” for glutamate as a weakness, the researchers showed that the experimental drug CB-839 stopped tumor growth in mice with KRAS and KEAP1 mutations by cutting off the cells’ glutamate supply. Because adenocarcinoma cells with mutations used up so much glutamate to enable antioxidant production, they did not have enough to fuel aggressive, KRAS-driven growth. Consequently, the cancer cells starved.
“Our study results suggest that a drug currently in clinical trials might be more effective against cancers with combined KRAS and KEAP1 mutations, which represent perhaps 10 percent of patients diagnosed with lung adenocarcinoma, or 9,000 patients per year,” says Dr. Papagiannakopoulos.
Beyond lung cancer, a second study published in October 2017 in eLife by the same team reported that KEAP1 mutations might also make glutaminase inhibitors such as CB-839 more effective against melanoma, bone cancer, kidney cancer, urinary tract cancer, and colon cancer, among others.