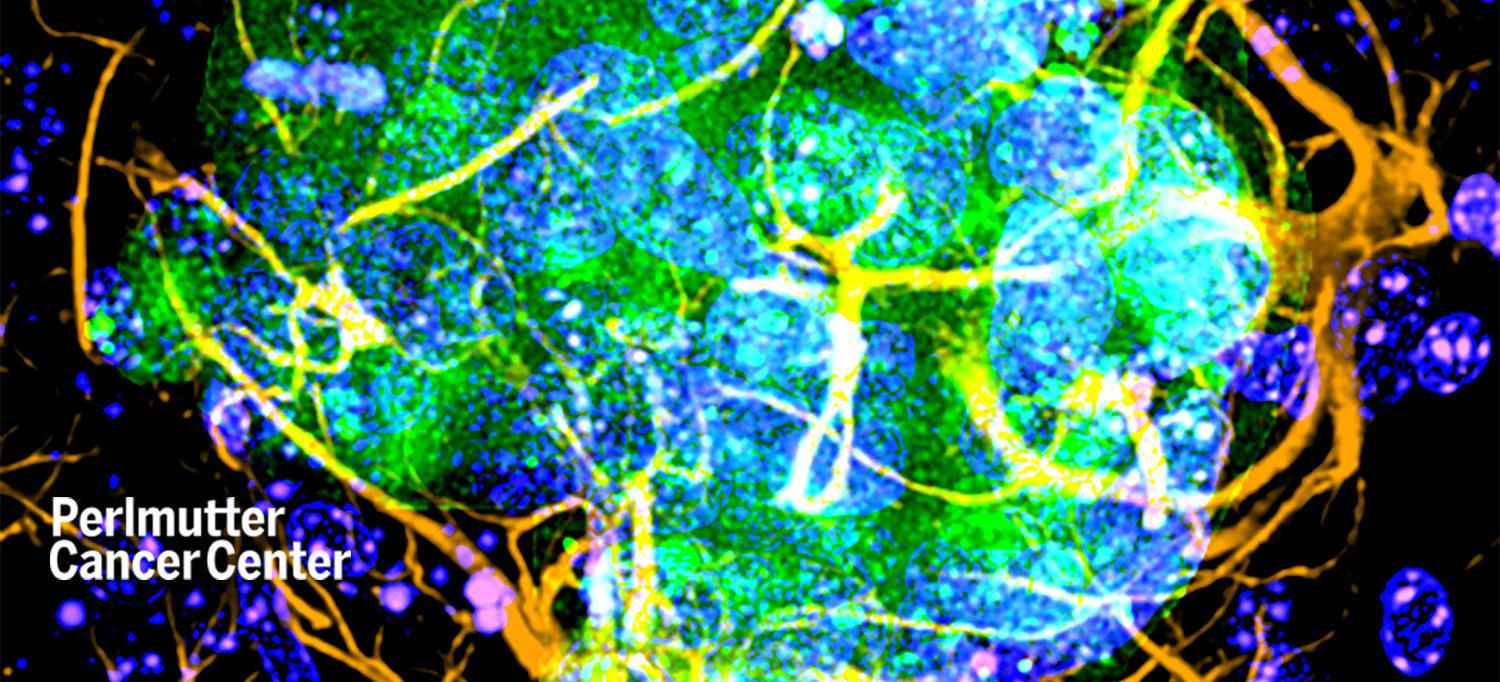
NYU Langone researchers have found that skin cancer cells, in green and blue, need amyloid beta, a protein associated with Alzheimer’s disease, to “take root” in the brain.
PHOTO: Copyright©2022 American Association for Cancer Research. All rights reserved.
Amyloid beta, a protein known to build up in the brains of people with Alzheimer’s disease, also helps skin cancer cells thrive when they spread to the brain, a new study finds.
Published online March 9 in Cancer Discovery, a journal of the American Association for Cancer Research, the study found that in melanoma, the deadliest form of skin cancer, cancer cells that have spread to the brain depend on amyloid beta to survive there. The study authors focused on melanoma because it spreads (metastasizes) to the brain in 40 percent of patients with advanced (stage 4) disease, the highest rate among common cancer types.
Led by researchers from NYU Grossman School of Medicine and NYU Langone’s Laura and Isaac Perlmutter Cancer Center, the study revealed that metastatic melanoma cells recovered from human brains and grown in tissue cultures make roughly three times as much amyloid beta as cancer cells that have spread to other parts of the body.
The research team also found that amyloid beta secreted by cancer cells ramps down immune responses that would otherwise recognize cancer cells as abnormal and attack them, much as they attack invading bacteria. The researchers theorize that amyloid beta shifts brain immune cells into a mode seen as infections fade and tissues begin to heal, enabling cancer cells to evade notice. In addition, the team showed that a treatment known to dramatically reduce amyloid beta levels, the beta secretase inhibitor LY2886721, decreased the size of brain melanoma metastases by about half in study mice.
“Our study reveals an unexpected role for tumor-secreted amyloid beta in promoting the survival of melanoma brain metastases, and suggests a new way to counter it,” says senior study author Eva M. Hernando-Monge, PhD, professor in the Department of Pathology and assistant dean for research integration.
The current finding adds to the mystery surrounding amyloid beta, the main component of deposits found in the brains of people with Alzheimer’s disease. Despite myriad studies, its roles in normal function and Alzheimer’s disease remain controversial, even as new proposed roles emerge, says Dr. Hernando-Monge, also a member of Perlmutter Cancer Center.
Cancer Can’t Take Root
The new work featured refinements on standard techniques that captured a more accurate picture of which proteins are made in greater levels in melanoma cells that have spread to the brain. First, the research team grew cells taken from the human metastatic brain tumors in cultures, but only for a short time to keep them from evolving genetically until they no longer resembled the original cancer cells. The authors then measured the proteins produced by the melanoma cells in the first use, to their knowledge, of a whole cell proteomics test to study brain metastases.
Using 24 human brain and non–brain cancer metastases grown in short term-cultures, the team was able to show that melanoma cells from the brain produce proteins related to Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease. The discovery of a connection between brain cancer and neurodegenerative diseases was made possible, say the authors, by new techniques that let the research team tell proteins made by cancer cells apart from those made in surrounding brain cells.
From these data, the researchers hypothesized that cancer cells produce amyloid beta in the brain to help their survival. To test the idea, they looked at the effect of silencing the gene that codes for amyloid precursor protein (APP), a protein that is processed by secretase enzymes (beta and gamma) into amyloid beta, in melanoma cells injected into the hearts of study mice. Silencing the APP gene, and therefore cutting off the amyloid beta supply from the cancer cells, dramatically reduced the amount of cancer metastases that formed in the brain, as measured by imaging.
Other experiments revealed that melanoma cells lacking amyloid beta became unable to successfully grow (divide and multiply) because of immune attack at the stage where they are forming small cell colonies (micrometastases) needed for spreading cancer cells to “take root” in a new tissue.
Finally, the study found that amyloid beta released by melanoma cells changes gene expression in astrocytes, brain cells that nourish message-carrying brain cells (neurons), such that the astrocytes emit proteins that ramp down immune responses to cancer. Astrocytes are also known to exchange signals with microglia, a type of immune cell in the brain. The researchers further demonstrated that amyloid beta released by melanoma cells prevents them from being destroyed by microglia. It may be that amyloid beta released by melanoma cells is influencing microglia, both through astrocytes and directly, to keep them from “swallowing” and destroying melanoma cells, say the authors.
“The field has already developed treatments that have been shown in clinical trials to potently and safely reduce amyloid beta levels, but that fail to counter Alzheimer’s disease for reasons unknown,” says first study author Kevin Kleffman, PhD, an MD/PhD student at NYU Langone and member of Dr. Hernando-Monge’s lab. “With this in mind, our team is already evaluating whether repurposed, tested anti–amyloid beta antibodies could prevent or reduce brain metastases in animal studies. Another next step is combining immunotherapies, including checkpoint inhibitors, and anti–amyloid beta therapies to ensure they can be used safely together.”
Along with Dr. Hernando-Monge and Dr. Kleffman, study authors in the Department of Pathology at NYU Grossman School of Medicine were Grace Levinson, Sorin Shadaloey, Francisco Galan-Echevarria, Alcida Karz, Diana Argibay, Richard Von-Itter, Alfredo Floristan, Gillian Baptiste, Nicole Eskow, Robert Rogers, and George Jour. Also NYU Langone Health authors were Indigo Rose and Shane Liddelow of the Neuroscience Institute, Lili Blumenberg and Kelly Ruggles in the Department of Medicine, Avantika Dhabaria and Beatrix Ueberheide in the Department of Biochemistry and Molecular Pharmacology; James Tranos, Jenny Chen, and Youssef Zaim Wadghiri in the Department of Radiology; Eleazar Vega Saenz de Miera, Melissa Call, and Iman Osman in the Ronald O. Perelman Department of Dermatology; Paul Mathews in the Department of Psychiatry, and Robert Schneider in the Department of Microbiology. Other authors were Eitan Wong and Yueming Li in the Chemical Biology Program at Memorial Sloan Kettering Cancer Center, and Ronald DeMattos of the Department of Neurobiologics at Eli Lilly.
The study was supported by National Institutes of Health (NIH) grants NCI 5R01CA243446, P01CA206980, NCI P50 CA225450, F30CA221068, 5 T32 CA009161-37, P30CA016087, NIGMS 5 T32 GM007308-41, and S10 Grants NIH/ORIP S10OD01058 and S10OD018338. Also supporting the work were an American Cancer Society–Melanoma Research Alliance Team Science Award, a Vilcek Foundation Scholarship, a Fundacion Ramon Areces fellowship, the Cure Alzheimer’s Fund, the Blas Frangione Foundation, the MD Anderson Neurodegenerative Consortium, anonymous donors, and NYU Grossman School of Medicine.
Of note, none of the study authors from NYU Grossman School of Medicine received any financial compensation from Eli Lilly, which supplied the beta secretase inhibitor used in the study. Study author Ronald DeMattos is a full-time employee at Eli Lilly. Eva Hernando-Monge, Robert Schneider, and Kevin Kleffman are inventors on pending International Patent Application No. PCT/US2019/033377 filed on May 21, 2019, for a method-of-treatment patent in the use of an anti–amyloid beta therapeutic. Shane Liddelow is a founder of AstronauTx Ltd, a company making therapies to target astrocytes in neurodegenerative disease. These relationships are being managed in accordance with the policies of NYU Langone Health.
Media Inquiries
Greg Williams
Phone: 212-404-3500
gregory.williams@nyulangone.org