Howard A. Riina, MD
PHOTO: John Abbott
Previously advised against surgery because of the risky location of his thalamic lesion, a patient found new hope in NYU Langone Health’s Center for Stroke and Neurovascular Diseases and an advanced cerebrovascular approach carefully coordinated by a multidisciplinary team.
In the 16 years since a right thalamic cavernous malformation was identified on MRI imaging, the patient had had multiple hemorrhages, a relatively common complication of thalamic cavernous malformations. In addition to expansion of the lesion, the patient had also experienced progressive hemisensory loss and dysesthetic pain syndrome, making treatment imperative. “The patient had been told by other surgical teams that the cavernous malformation was inoperable, because there was increasing concern about progressive functional damage,” says Howard A. Riina, MD, professor of neurosurgery, radiology, and neurology. “It was critical to find a surgical approach that could help mitigate potential risks, including left hemiplegia and venous infarction of the thalamus.”
Preoperative Planning and Targeted Mapping Set Trajectory for Success
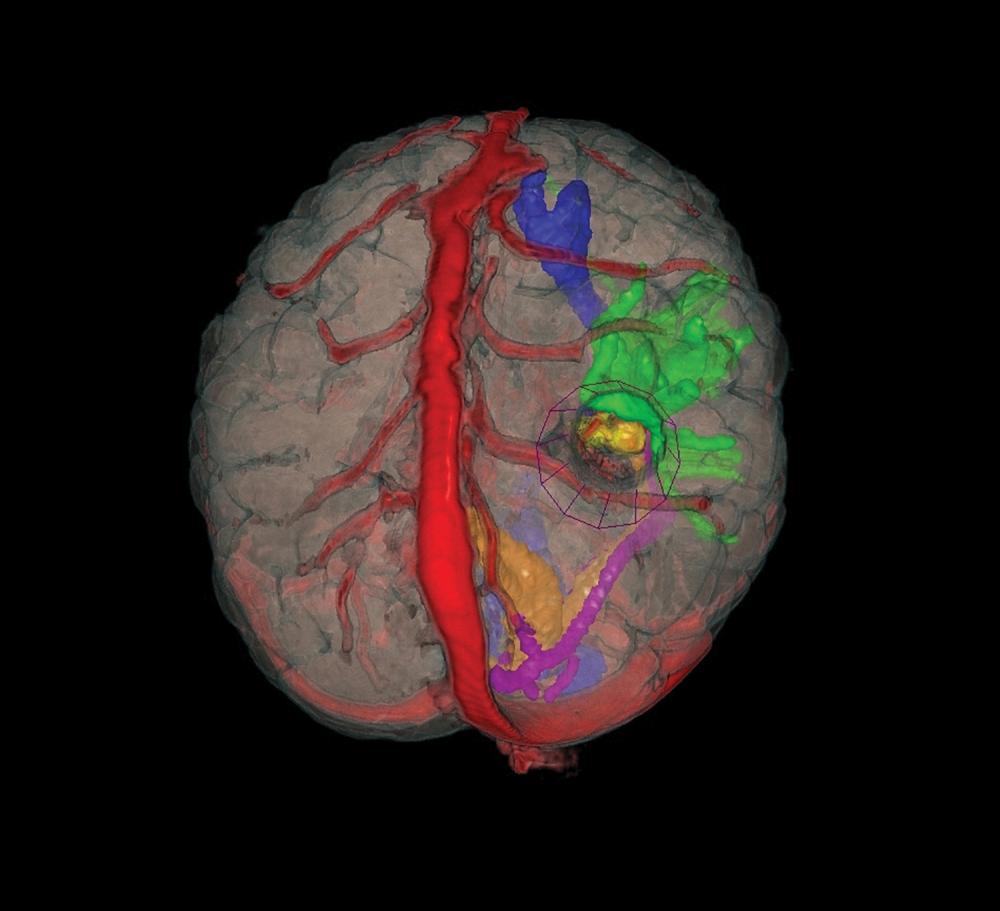
The optimal resection approach for thalamic cavernous malformation depends on the location of the malformation in the thalamus. This patient’s malformation was in the lateral posteroinferior region, bordered anteriorly by the medial and lateral thalamus, so that a parieto-occipital transventricular approach through the superior parietal lobe could be used to bypass the optic radiations and the somatosensory region.
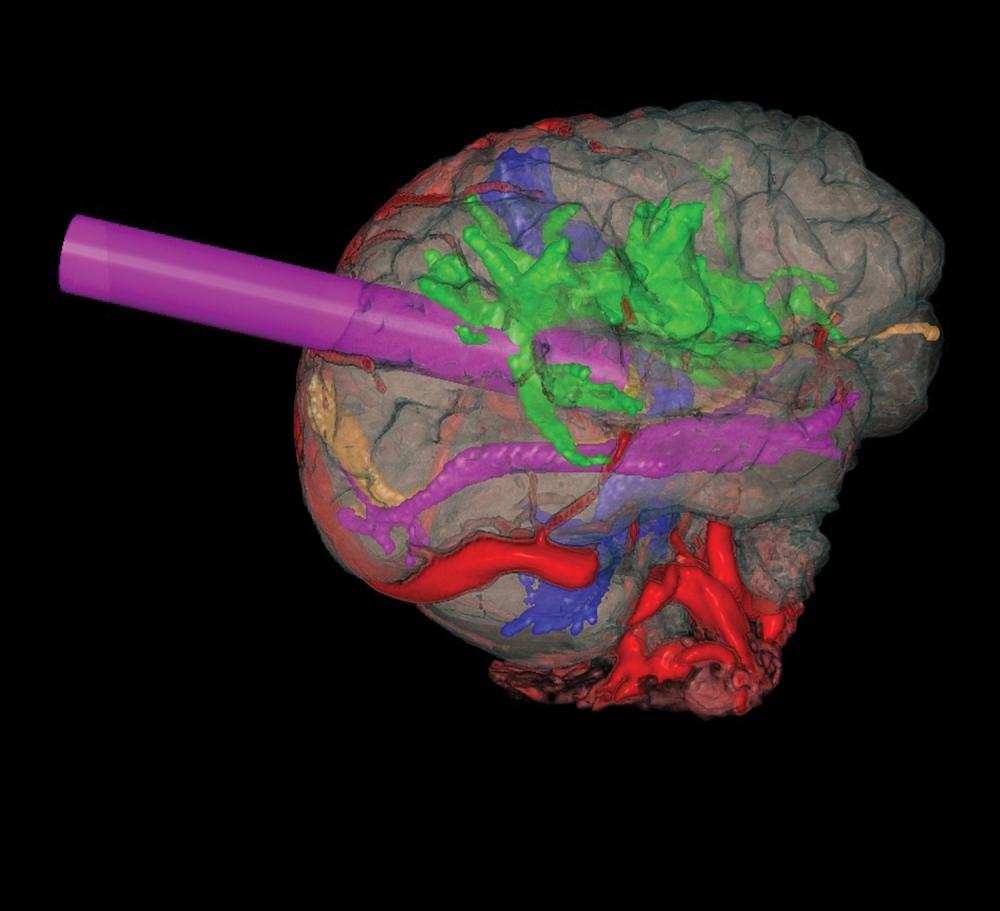
“We decided that the best way to access the malformation safely was to perform the procedure through a small tube, a minimally invasive approach typically used for brain tumors,” explains Dr. Riina, who handled the cerebrovascular aspects of the surgery. “Rather than cut into the thalamus, we found a very simple trajectory through the ventricle, from which I could extend into the thalamus to gain access to the lesion.”
Advanced imaging was imperative to guide the surgeons’ precision approach. Using Surgical Theater’s surgical planning software to combine MRI, MR angiography, and diffusion tensor imaging, the team selected a target point. The combination of the data sets into a three-dimensional surgical planning model allowed the surgeons to map multiple trajectories and choose the best one: through the posterior medial parietal lobe, superior to the optic radiations and posterior to the somatosensory cortex, to the lesion. The borders of the cavernous malformation were simultaneously drawn in the Brainlab image guidance system to create a lesion volume around the target. This combined approach, using surgical planning software and image guidance, provided surgical access that transversed the least amount of brain tissue, significantly reducing the risk of complications and permanent injury.
Intraoperative Projections Improve Surgical Precision
With planning in place, Dr. Riina was joined in the operating room by John G. Golfinos, MD, associate professor of neurosurgery and otolaryngology and chair of the Department of Neurosurgery, and Jeffrey H. Wisoff, MD, professor of neurosurgery and pediatrics. Continuous stereotactic guidance and tractography supported the procedure’s navigation, while Surgical Theater and Brainlab projections oriented the surgeons to the limits of the tumor, confirmed the approach vector, and guided the placement of the tubular retractor. The surgeons worked as a team throughout the procedure, with each surgeon rotating in at critical junctures to lend his specific expertise.
After performing a circular craniotomy, the dura was opened in cruciate fashion. The operating microscope was introduced through a prominent sulcus selected as the entry point. To displace delicate tissues and folds of the brain—and limit intraoperative damage—a tubular retractor was advanced along the chosen trajectory into the atrium of the lateral ventricle, entering the thalamus at a point identified by the microscope and pointer as superior and lateral to the choroid plexus. Precise navigation for microdissection was enabled by visual tracking.
Immediately after making a small corticectomy, the surgeons encountered hemosiderin staining within the tissue. A large developmental venous anomaly was identified medially and preserved intact. “The lesion was only a few millimeters deep to the surface, just as we had seen in presurgical imaging,” Dr. Riina recalls. “We worked in turns to resect the cavernous malformation and evacuate old hemorrhage products so that we could ensure that we reached the borders in all planes.”
“This is a remarkable outcome given the lesion’s treacherous location and the precision approach it demanded.”— John G. Golfinos, MD
The thorough excision of the malformation was enabled by comprehensive, imaging-aided preplanning that allowed the surgical team to choreograph the removal without leaving a trace—and set the patient on a swift path to recovery. “Two days after the operation, the patient went home,” reports Dr. Golfinos. “This is a remarkable outcome given the lesion’s treacherous location and the precision approach it demanded.”
Multidisciplinary Collaboration and Concentrated Resources Enhance Neurovascular Care
This achievement, notes Dr. Riina, is a credit to the collaboration and teamwork fostered by experts in the Center for Stroke and Neurovascular Diseases. “These lesions would be extraordinarily difficult for surgeons to treat without the concentration of advanced neuroimaging, protocols, and neurosurgical expertise available at NYU Langone,” he adds. “Our center is unique in that respect, giving patients with unique and complex cases options for successful surgical treatment that are simply unavailable elsewhere.”