Center for Cognitive Neurology researchers Dr. Thomas Wisniewski and Dr. Allal Boutajangout are focusing on developing targeted immunotherapies for treating Alzheimer's disease.
Photo: Karsten Moran
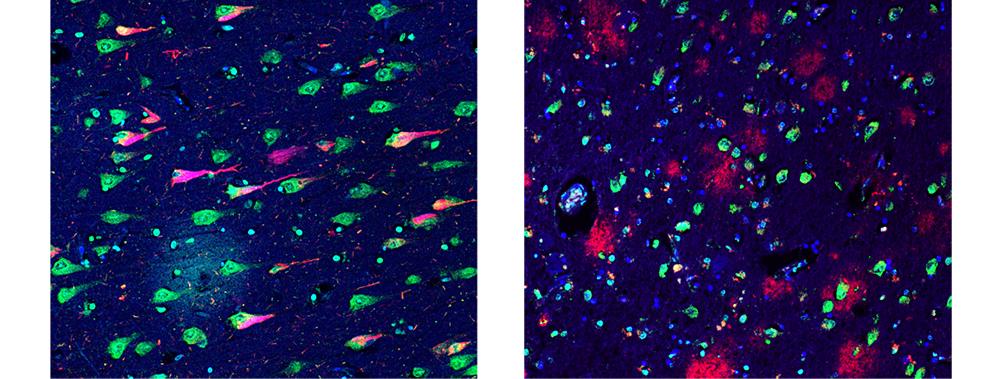
Researchers at the Center for Cognitive Neurology continue to focus on therapies for Alzheimer’s disease, targeting the condition’s molecular roots and isolating highly neurotoxic molecules to reduce pathology without evoking toxic side effects.
By isolating neurotoxic molecules and creating synthetic oligomers, NYU Langone Health researchers have advanced their search for new leads in targeted immunotherapy for Alzheimer’s disease.
The researchers’ investigations, described in two 2018 articles, use antibodies that attack the characteristic beta sheet structure created when monomers combine into oligomeric form—a key step in Alzheimer’s disease progression.
Because this beta sheet structure is shared by many types of oligomers, these antibodies can be used to target toxic proteins, including the amyloid beta and tau proteins that predominate in the brains of patients who have Alzheimer’s disease.
These studies build on the researchers’ original animal studies, published in 2017 in Scientific Reports, which demonstrated that oligomer-fighting antibodies could be created from p13Bri, a protein associated with the rare genetic disease British amyloidosis, and then be used to target the structure of the oligomers, rather than the proteins, to avoid a toxic autoimmune response.
“These antibodies offer a more focused approach compared with existing immunotherapy treatment options for Alzheimer’s disease,” says Thomas M. Wisniewski, MD, the Gerald J. and Dorothy R. Friedman Professor of Neurology, professor of pathology and psychiatry, and director of the Center for Cognitive Neurology. “In addition to their reduced risk of toxic side effects, they allow the simultaneous targeting of multiple pathologies and proteins—versatility that is vital considering the variable characteristics of dementia-causing pathology from person to person.”
New Studies Affirm the Effectiveness of Oligomer-Targeting
In 2018, Dr. Wisniewski and colleagues published a pair of animal studies in Alzheimer’s Research & Therapy that evaluated the efficacy of two monoclonal antibodies developed in the lab.
The drugs, GW-23B7 and TWF9, both significantly reduced oligomeric forms of amyloid beta and tau proteins and also improved cognitive performance in aged mice with extensive Alzheimer’s disease pathology.
“In both studies, we began treatment in animals whose Alzheimer’s disease was quite advanced,” says Dr. Wisniewski. “In many previously published immunotherapeutic approaches, the antibodies were given at the onset of disease pathology, but we were seeking to demonstrate therapeutic efficacy in late-stage disease.”
Researchers plan to test the monoclonal antibodies in nonhuman primates and are submitting grant proposals for phase I human trials of the drugs.
Researchers at the center also plan to expand their approach to other neurodegenerative disorders. One investigation will examine the impact of the antibodies on traumatic brain injury, where neurodegeneration appears to be driven mainly by toxic tau protein. Another will test their approach in Parkinson’s disease—which, along with Lewy body disease, is caused primarily by alpha synuclein oligomers—and a third investigation will study diseases linked to prions, such as mad cow disease.
Researchers are also collaborating with neuroradiologists to examine the effectiveness of a novel imaging tool to complement this oligomer-targeting drug development approach. The new technology uses a ligand that binds to oligomers, allowing them to be visualized on PET scans. “This ligand could enable us to visualize and quantify the oligomeric species in animal models as well as in Alzheimer’s disease patients themselves,” notes Dr. Wisniewski.
Additional Immunological Investigations Hold Promise for Alzheimer’s Disease
Researchers continue to advance investigations into other promising Alzheimer’s disease drugs, including CpG ODN, a novel molecule developed at NYU Langone that boosts activity of the brain’s primary immune cells by stimulating the immune system–mediating protein toll-like receptor 9 (TLR9).
“We've completed one full set of primate experiments, treating squirrel monkeys for two and a half years with this TLR9 agonist,” says Dr. Wisniewski, “and we’re seeing significant cognitive benefits and a reduction in Alzheimer’s disease-related pathology without any evidence of toxicity.” A second set of grant-funded primate experiments is underway, and application for funding for a human phase I clinical trial is planned.
There are also additional trials focused on immunotherapy and Alzheimer’s disease already in progress. Researchers are conducting phase III trials of aducanumab, a monoclonal antibody that is derived from healthy aged donors with no cognitive impairment and that appears to be effective at reducing amyloid plaque in the brains of patients with early Alzheimer’s disease. In addition, NYU Langone will be part of an upcoming TANGO study, a phase II trial of another novel immunotherapy agent, BIIB092, which targets tau proteins in the brain.