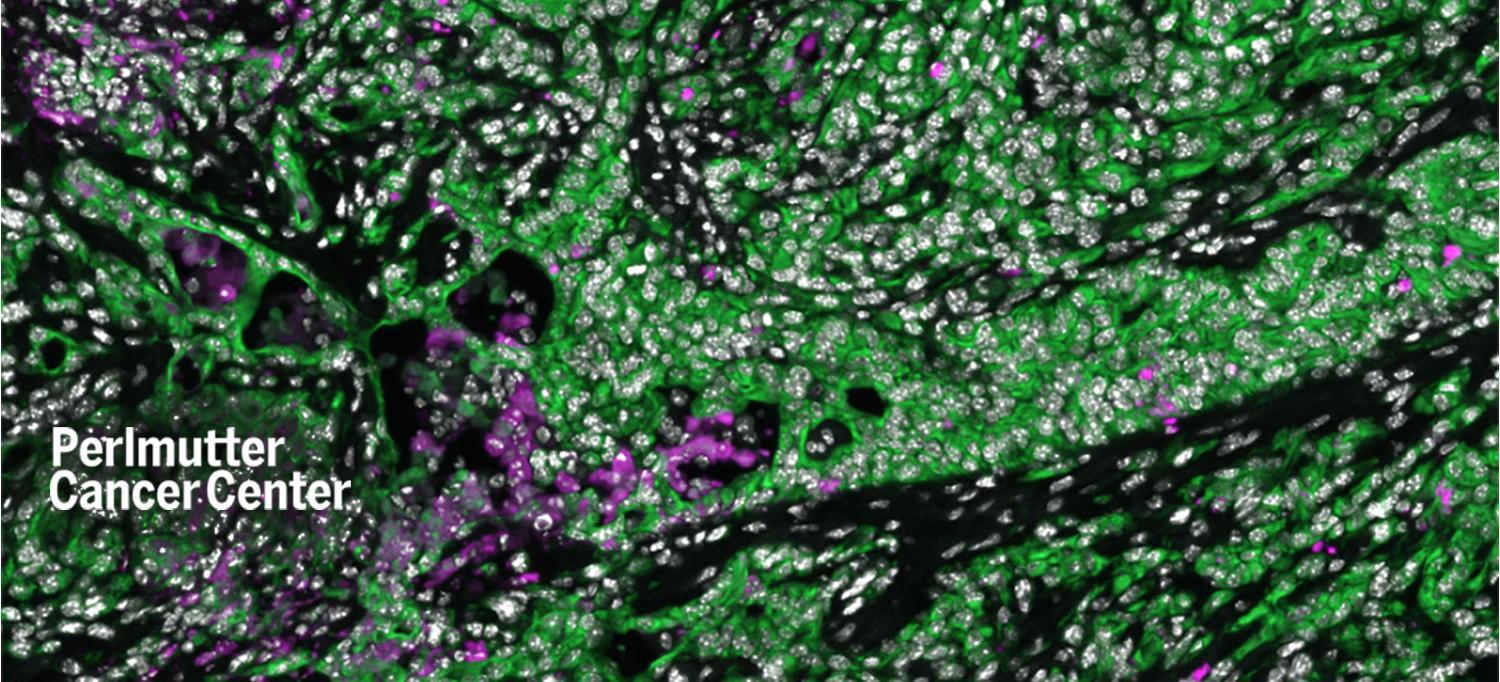
Pancreatic cancer cells are shown in green. Pink cells represent cancer cells that died in greater numbers in mice after they underwent aerobic exercise.
IMAGE COURTESY OF NYU LANGONE HEALTH
Aerobic exercise reprograms the immune system to reduce pancreatic tumor growth and amplify the effects of immunotherapy, a new study finds.
Published online in Cancer Cell June 2, the study provides new insight into how the mammalian immune system, designed to attack foreign invaders like bacteria, can also recognize cancer cells as abnormal. Exercise-induced increases in levels of the hormone adrenalin cause changes to the immune system, say the study authors, including in the activity of cells that respond to signaling protein interleukin-15 (IL-15).
Biological systems that fight disease and repair tissue are intertwined, the researchers say, with IL-15 signaling, based on the context, either encouraging the recovery of muscles after exercise or, in the case of the current work, amplifying immune attack on pancreatic cancer cells.
Led by researchers at NYU Grossman School of Medicine and NYU Langone’s Perlmutter Cancer Center, the current study found that exercise promotes the survival of CD8 T cells sensitive to IL-15, and doubles the number of them homing to pancreatic ductal adenocarcinoma (PDAC) tumors in mice. Such “effector” T cells have been shown by other studies to be capable of killing cancer cells. Other tests found that aerobic exercise for 30 minutes 5 times a week reduced the rate of cancer formation by 50 percent in one mouse model of PDAC, and reduced tumor weight by 25 percent in another model, in which mice ran on treadmills for 3 weeks.
In collaboration with The University of Texas MD Anderson Cancer Center, the study authors then found that human patients enrolled in their Preoperative Rehabilitation During Neoadjuvant Therapy for Pancreatic Cancer clinical trial who exercised before surgery to remove their pancreatic tumors had more CD8 effector T cells that expressed a protein called granzyme B, which confers tumor cell–killing ability. Also in that trial, which opened in 2017, those patients who exercised and had more of these cell types had 50 percent higher overall survival over 5 years than patients with fewer of them.
“Our findings show, for the first time, how aerobic exercise affects the immune microenvironment within pancreatic tumors,” says first author Emma Kurz, MD, PhD, a graduate student in the Molecular Oncology and Tumor Immunology PhD Training Program at NYU Grossman School of Medicine’s Vilcek Institute of Graduate Biomedical Sciences. “The work helped to reveal that activation of IL-15 signaling in pancreatic cancer might be an important treatment approach in the future.”
Boosting Therapeutic Response
In the last several years, as the role of the IL-15 signaling in tumors became clear, other researchers attempted to treat cancer by direct infusion of this protein, which unfortunately increased the risk of systemic inflammatory damage. Subsequently, the field designed treatments based on the fact that signaling proteins such as IL-15 fit into receptors proteins (IL-15Rα), like a key into a lock, on the surface of target T or NK cells. New drug candidates mimic these “lock and key” interactions, which transmit a message to activate the target cell.
The pharmaceutical company Novartis has been developing a “superagonist” agent, NIZ985, which is designed to enhance IL-15/IL-15Rα pathway signals with reduced potential for harmful inflammatory effects. This approach has not yet been tested in large numbers of people with pancreatic cancer.
In the current study, Dr. Kurz and colleagues showed that either aerobic exercise or treatment with NIZ985 increased the effectiveness of chemotherapy and an existing treatment that blocks the effect of a protein called protein death receptor 1 (PD-1) in mice. To spare normal cells from immune attack, the immune system uses “checkpoints,” like PD-1 sensors, on immune cells that turn them off when they receive the right signal. Cancer cells hijack such checkpoints to inactivate immune responses.
Drugs that block the function of PD-1 can make tumors “visible” again to immune cells, but have had little efficacy against PDAC, which has a 5-year survival rate of 10 percent. The Perlmutter Cancer Center research team found that PD-1 blockade increased the number of IL-15–responsive, cancer cell–killing CD8+ T cells in tumors of mice by 66 percent alone, but by 175 percent when combined with exercise. In addition, the authors found that combination of IL-15 superagonist NIZ985 and PD-1–inhibiting therapy increased the survival of mice with advanced pancreatic cancer by 100 percent.
“Our work demonstrates that exercise, and related IL-15 signals, can prime treatment-resistant pancreatic tumors for improved responses to immune-based therapeutics,” says study senior author Dafna Bar-Sagi, PhD, senior vice president, vice dean for science, and chief scientific officer at NYU Langone Health. “That even mild exercise can profoundly alter the environment in tumors points to the potential of this approach in treating patients with a devastating disease burden and few options.”
As a result of the current work, the study team is collaborating with Perlmutter Cancer Center member Paul E. Oberstein, MD, director of gastrointestinal oncology at NYU Langone, as well as members from Rusk Rehabilitation, to launch a clinical trial assessing the immune effects of exercise in people with pancreatic cancer. In addition, the team plans to continue exploring the potential efficacy of IL-15 superagonists in combination with chemotherapy to combat pancreatic tumors.
Along with Dr. Kurz and Dr. Bar-Sagi, study authors from the Departments of Cell Biology and Biochemistry and Molecular Pharmacology at NYU Langone were Carolina Alcantara Hirsch, Sorin Alberto Shadaloey, and Emily Vucic, along with Alireza Khodadadi-Jamayran from the Applied Bioinformatics Laboratories and Rafael Winograd of Perlmutter Cancer Center. Also an author was Tanner Dalton in the Department of Cell Biology at Columbia University’s Irving Medical Center. Collaborators from Moffitt Cancer Center and MD Anderson Cancer Center included Sumedha Pareek, Hajar Rajaei, Maria Petzel, Chirayu Mohindroo, Seydra Baydogan, An Ngo-Huang, Nathan Parker, Matthew HG Katz, Keri Schadler, and Florencia McAlister.
The work was funded by National Cancer Institute (NCI) grants P30CA16087, S10 OD021747, F30 CA243205, T32-GM136573, and CA210263; as well as by National Center for the Advancement of Translational Science (NCATS) grant UL1 TR000038, Perlmutter Cancer Center Support Grant P30CA016087, the Wyck Knox Jr. Family Foundation, the Center for Energy Balance in Cancer Prevention & Survivorship, Duncan Family Institute for Cancer Prevention and Risk Assessment, Cancer Prevention & Research Institute of Texas (CPRIT) Training Grant/MD Anderson Cancer Prevention Research Training Program (RP170259), the NIH/NCI (R21CA218732 and P30CA016672), NCI (R37CA237384–01A1), CPRIT (RP200173), and a Canadian Institutes of Health Research Fellowship.
The IL-15 superagonist agent (NIZ985) used in the study was provided by Novartis. None of the NYU Langone authors received financial compensation from Novartis. The study collaboration is being managed in keeping with the policies of NYU Langone Health.
Media Inquiries
Greg Williams
Phone: 212-404-3500
gregory.williams@nyulangone.org

