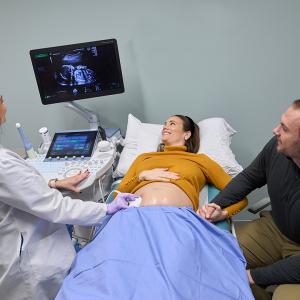
Kelsey Kim with her husband, Dan, and their daughters Marigold, Abigail, and Ruby.
Courtesy of Kelsey Kim
In the summer of 2024, Kelsey and Dan Kim, from Falls Church, Virginia, faced an agonizing decision: whether or not to have another child. They had already lost a son, Arthur, to neonatal lupus erythematosus, an autoimmune disorder caused by maternal proteins called autoantibodies that cross the placenta during pregnancy and attack the fetal heart. These proteins can cause congenital heart block, a condition in which the organ’s electrical conduction system is scarred and beats too slowly. Arthur died two years earlier from heart block during the 22nd week of gestation, despite emergency measures.
During her next pregnancy, in 2023, Kelsey, had been closely monitored and treated by her doctors in Washington, DC. Even so, congenital heart block struck again in the 18th week. “This time, treatment was effective in holding off the worst of the damage,” she recalls. When baby Abigail was born, though, she had to have a pacemaker implanted to improve her dangerously slow heart rate.
A decade earlier, Kelsey herself had been diagnosed with a mild form of lupus after experiencing rashes under her eyes and joint pain and swelling in her hands. Medication kept those symptoms in check. However, tests revealed the presence of harmful anti-SSA/Ro antibodies, which in some women cross the placenta and injure the fetal heart. The Kims desperately wanted to expand their family of four, including Abigail, age 2, and her older sister, Ruby, 6, who had no complications beyond a temporary ring-shaped rash characteristic of neonatal lupus. But Kelsey and Dan were fully aware that another pregnancy could mean more heartbreak.
Ultimately, they decided to hope for the best. During Kelsey’s ninth week of pregnancy, in November 2024, Angus Worthing, MD, her rheumatologist in Washington, recalled hearing a lecture by Jill Buyon, MD, director of the Lupus Center at NYU Langone Health. His call to Dr. Buyon set in motion a novel test of an experimental drug to determine whether it could prevent the transfer of Kelsey’s harmful autoantibodies.
A previous study by Dr. Buyon and colleagues had shown that anti-SSA/Ro antibodies are an essential contributor to congenital heart block, which is fatal in about 20 percent of cases, but not the entire cause. While her lab is seeking to identity other disease contributors, the findings pointed to a therapeutic strategy that Dr. Buyon has long pondered. “Understanding what the antibody is doing in the fetal heart is obviously very important science, but what if you eliminated the antibody?” she asks. “Basically, this study was born with the idea ‘No antibody, no disease.’ The concept behind that has been on my mind for 30 years.”
Dr. Buyon, the Sir Deryck and Lady Va Maughan Professor of Rheumatology and director of the Division of Rheumatology at NYU Grossman School of Medicine, got the chance to test her hypothesis when, in June 2023, the FDA approved a drug called rozanolixizumab to treat the autoimmune disorder myasthenia gravis. The successful clinical trial for that disease, along with other research, showed that the new medication reduces the overall levels of IgG antibodies and autoantibodies involved in myasthenia gravis. She and her team hoped that the drug would lower anti-SSA/Ro antibodies as well as block their transport across the placenta. When Kelsey volunteered to be the first and only trial participant to see whether rozanolixizumab might similarly block the antibodies contributing to congenital heart block, Dr. Buyon obtained a compassionate-use authorization to treat her.
Every Monday for 14 weeks, starting during her 14th week of pregnancy, Kelsey took the train from Washington to New York for an injection of the drug. While Dr. Buyon monitored her lupus symptoms, Justin S. Brandt, MD, director of the Division of Maternal–Fetal Medicine, helped manage her high-risk pregnancy. Kelsey also tracked her baby’s heartbeat at home with a fetal monitor and regularly saw Dr. Worthing and Mary Donofrio, MD, her pediatric cardiologist at Children’s National Hospital.
Kelsey had no significant side effects from the drug, but the potential risk of infection required a careful determination of when to stop administering it. With no signs of congenital heart block by Kelsey’s 28th week of pregnancy—comfortably beyond the high-risk period of 17 to 25 weeks—the clinical team discontinued her weekly injections.
“We were cautiously optimistic once the most vulnerable period passed,” says Dr. Brandt, “but we did not fully exhale until the baby was born without heart block or any signs of cardiac neonatal lupus.” That good news came on June 10, when Kelsey gave birth to Marigold, a healthy baby girl.
In a study published recently in the Annals of the Rheumatic Diseases, Dr. Buyon, Dr. Brandt, NYU Langone rheumatology fellow Philip Carlucci, MD, and colleagues described the experimental intervention and successful outcome. Dr. Buyon acknowledges that it’s impossible to know for sure whether the result was due to the blocked transfer of maternal antibodies or some other reason. Even so, she credits the completion of the landmark study, funded in part by Lauren and Andrew Levison, to NYU Langone’s strong emphasis on translational research, a process that aims to accelerate the journey from the laboratory to patient care. “Our dedication to women’s health and to helping families grow drove us to take a reasonable risk on a drug that had never been used in a pregnant woman, for a couple that desperately wanted to have a heart-healthy child,” notes Dr. Buyon.
Building on their close partnership, Dr. Buyon and Dr. Brandt teamed up with University of Arizona pediatric cardiologist Bettina Cuneo, MD, to propose a multicenter trial to test the drug’s efficacy on a larger pool of patients with lupus during their high-risk pregnancies. The National Institutes of Health recently awarded a $3.7 million, five-year grant to fund the study.
Kelsey says she feels immense gratitude to NYU Langone and finds it rewarding that her family’s experience helped pave the way for a trial that could help others as well. “The process of growing our family has been a painful one,” she says. “So for me, being able to do something that might spare other families from going through what we did is a big deal.”