Daily Fetal Heart Rate Monitoring More Helpful in Certain Cases
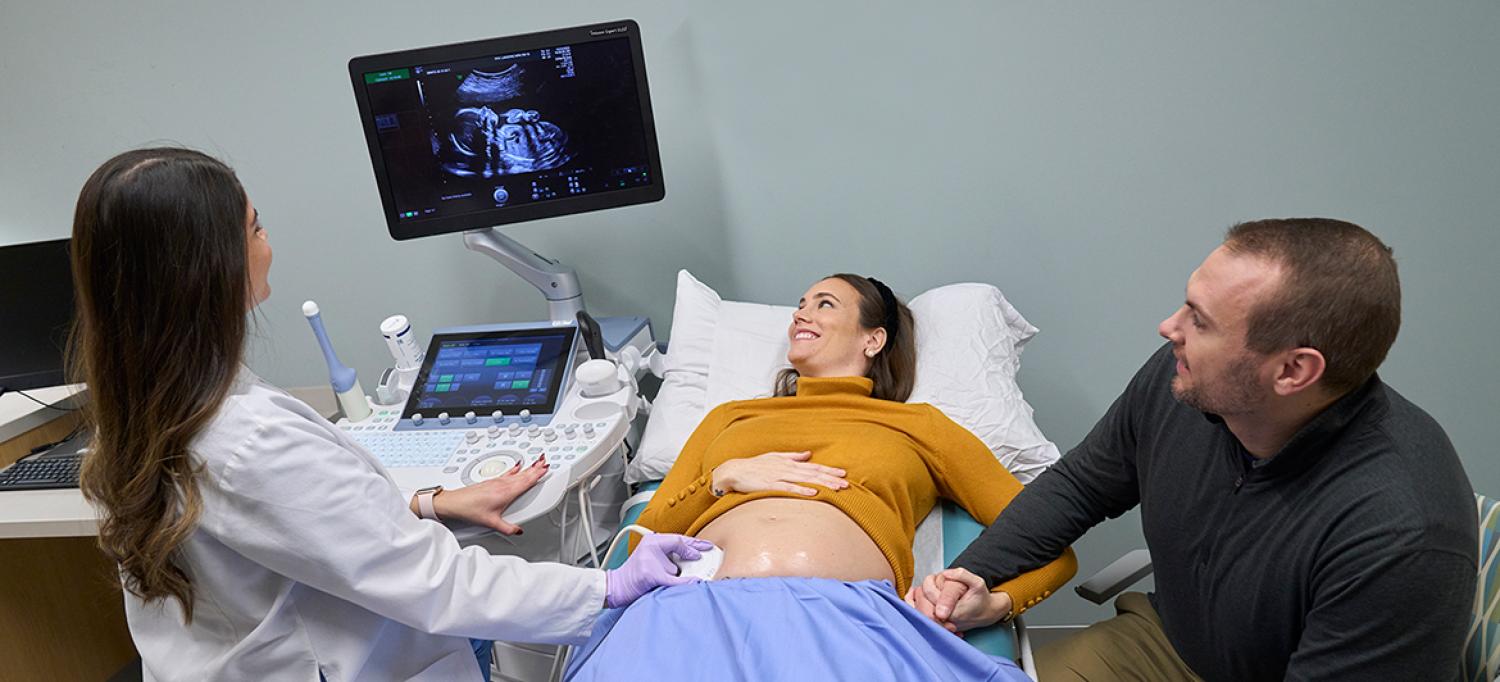
The new study shows that daily fetal heart rate monitoring best serves pregnant women with relatively higher blood levels of anti-SSA/Ro antibodies.
Photo: Juliana Thomas
Physicians who care for pregnant women have long sought to identify which of their patients would most likely benefit from monitoring of their fetus’s heart rate to catch early signs of their unborn child developing heart problems tied to the mother’s immune system. Slowed heart rates are often an initial symptom of abnormal electrical rhythms that indicate a mother’s immune system antibodies are attacking the fetus’s healthy heart tissue, much as these antibodies would attack an invading virus. The fetuses of pregnant women with lupus erythematosus, Sjogren’s syndrome, or other autoimmune diseases—or even totally without any symptoms of autoimmunity who have the culprit anti-SSA/Ro antibodies—are most at risk, experts say.
Now, a new study led by researchers at NYU Grossman School of Medicine, in collaboration with 21 other medical centers in the United States and Canada, has concluded that daily fetal heart rate monitoring best serves pregnant women with relatively higher blood levels of anti-SSA/Ro antibodies.
Preliminary findings from the study, which was designed to learn if heart rhythm problems could be detected and treated early during pregnancy, showed that pregnant women with low anti-SSA/Ro blood levels may be able to forgo daily monitoring, which can be stressful, time-consuming, and costly for both pregnant women and caregivers. Rhythm defects, often known as cardiac neonatal lupus and tied to the presence of anti-SSA/Ro, are rare, the researchers say, affecting an estimated 1 in 20,000 infants. But they can lead to irreversible and potentially deadly heart rhythms, such as atrioventricular block.
Researchers say efforts to predict which pregnancies are most at risk of fetal heart block are urgent because permanent damage to the fetal heart can progress quickly from early disease, which the researchers hope to show can be reversed with therapy. Death may occur during pregnancy or after birth, and in most survivors, a slowed or irregular heart rate requires the insertion of a pacemaker at birth or later in life to increase the heartbeat. Others may need a heart transplant or die prematurely of cardiac complications.
Monitoring occurred during the second trimester of pregnancy (from 17 weeks through 26 weeks) and consisted of daily fetal home testing by mothers using handheld, commercially available Doppler devices. These were followed up by weekly or biweekly echocardiograms, detailed ultrasound tests that can be used to evaluate fetal heart rhythm and function, as well as to show any structural problems. Three times per day, Doppler sound recordings were texted by the pregnant women in real time to a pediatric cardiologist, who in cases of irregular or slowing fetal heart rates immediately ordered an additional echocardiogram. If heart block was detected, drug therapy was initiated.
Among the 413 pregnant women who volunteered for the study and whose blood tested positive for anti-SSA/Ro antibodies, 45 Doppler recordings out of the more than 30,000 sent were suspected of being abnormal and then referred for urgent echocardiogram testing. Ten cases of heart block were confirmed, with seven considered as early-stage heart block and started on treatment. All seven were diagnosed by echocardiogram within five hours of initial detection of an abnormal Doppler recording.
None of the 10 were among pregnant women whose blood anti-SSA/Ro antibody levels were below a threshold of 1,000 ELISA units per milliliter. Indeed, 6 out of 10 cases of heart block were in mothers-to-be with extremely high blood anti-SSA/Ro antibody levels above 19,600 ELISA units per milliliter (the highest quartile of measurements). ELISA is a test for antibodies.
“Our preliminary study results show that ‘low’ and ‘high’ blood levels of anti-SSA/Ro antibodies are an effective tool for determining which pregnancies are most at risk of resulting in a case of neonatal lupus,” said study co-lead investigator Jill P. Buyon, MD, the Sir Deryck and Lady Va Maughan Professor of Rheumatology in the Department of Medicine and the director of the Division of Rheumatology and the Lupus Center at NYU Langone.
“Our study findings demonstrate that women with low blood anti-SSA/Ro antibody levels may be able to safely forgo the cost, stress, and time needed for daily monitoring and routine ultrasound surveillance, with such screening reserved for only those with high blood anti-SSA/Ro antibody levels,” said Dr. Buyon. “Home monitoring of the fetal heart rate, backed up by specialist care, is a safe and low-cost means of surveillance by pregnant women whose unborn children are most at risk of developing heart block.”
Dr. Buyon says that further research is needed to determine what factors other than blood anti-SSA/Ro antibody levels contribute to the heart damage, which, she emphasized, is exceedingly rare. But the initial findings from the study, known as STOP BLOQ, short for Surveillance and Treatment to Prevent Fetal Atrioventricular Block Likely to Occur Quickly, are sufficient to establish a potential benchmark for universal screening of pregnant women for anti-SSA/Ro antibodies and treatment guidelines for the monitoring and screening for cardiac neonatal lupus. Dr. Buyon notes that the interpretation of home-monitoring recordings could in the future be made easier by application of artificial intelligence tools designed to prioritize which recordings need to be interpreted by a physician and are in need of an urgent echocardiogram.
Dr. Buyon and the STOP BLOQ team are presenting the study findings at the annual meeting of the American College of Rheumatology on November 13 in San Diego. The study results will be published in the organization’s journal Arthritis & Rheumatology.
Funding support for the study was provided by National Institutes of Health grants R01HD100929, N01AR042220, and P50AR070591.
Besides Dr. Buyon, Bettina Cuneo, MD, a research professor at the University of Arizona in Tucson, is the study co-senior investigator.
In addition to Dr. Buyon, other NYU Langone researchers involved in this study are co-lead investigators Mala Masson, BA, and Caroline Izmirly; co-investigators Colin K. Phoon, MD; Philip Carlucci, MD; Peter M. Izmirly, MD; Nicola Fraser, BS; and co-senior investigator Robert M. Clancy, PhD.
Other study investigators include Ruben Acherman, MD, at the Children’s Heart Center Nevada in Las Vegas; Elena Sinkovskaya, MD, and Alfred Abuhamad, MD, at Eastern Virginia Medical School in Norfolk; Majd Makhoul, MD, at Atrium Health Levine Children’s Hospital in Charlotte, North Carolina; Gary Satou, MD, at the University of California, Los Angeles; Whitnee Hogan, MD, and Nelangi Pinto, MD, at the University of Utah in Salt Lake City; Anita Moon-Grady, MD, at the University of California, San Francisco; Lisa Howley, MD, at Midwest Fetal Care Center in Minneapolis; Mary Donofrio, MD, and Anita Krishnan, MD, at Children’s National Hospital in Washington, DC; Homa Ahmadzia, MD, at George Washington University in Washington, DC; Stephanie Levasseur, MD, at Columbia University in New York City; Erin Paul, MD, at Mount Sinai Hospital in New York City; Sonal Owens, MD, at the University of Michigan in Ann Arbor; Kristopher Cumbermack, MD, at the University of Kentucky in Lexington; Jyothi Matta, MD, at the University of Louisville in Kentucky; Gary Joffe, MD, at Perinatal Associates of New Mexico in Albuquerque; Christopher Lindblade, MD, at Phoenix Children’s Hospital in Arizona; Caitlin Haxel, MD, at the University of Vermont Children’s Hospital in Burlington; Katherine Kohari, MD, and Joshua Copel, MD, at Yale University in New Haven, Connecticut; James Strainic, MD, at University Hospitals Rainbow Babies and Children’s Hospital in Cleveland; Tam Doan, MD, Karla Bermudez-Wagner, MD, Conisha Holloman, MD, and Sunil Sheth, MD, at Baylor College of Medicine in Houston; Stacy Killen, MD, at Vanderbilt University in Nashville, Tennessee; Theresa Tacy, MD, and Michelle Kaplinski, MD, at Stanford University in Palo Alto, California; and Lisa Hornberger, MD, at Stollery Children’s Hospital in Edmonton, Canada.
Media Inquiries
David March
Phone: 212-404-3528
David.March@NYULangone.org

