Results Could Explain Why Drugs Designed to Remove Amyloid Deposits Have Failed to Stop Disease
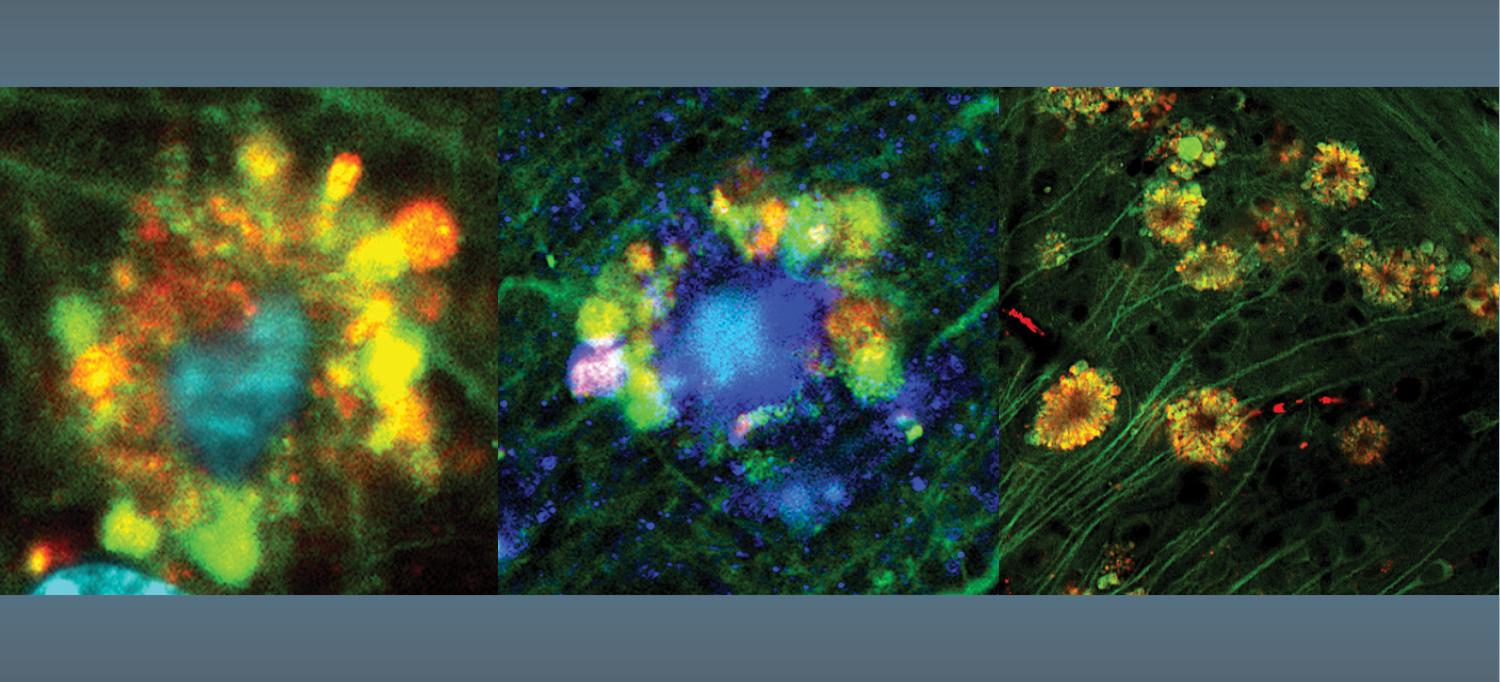
Fluorescent microscopy shows flower-like formations (at decreasing resolution) of autophagic vacuoles in neurons of a mouse with Alzheimer’s disease.
Photo: Courtesy of Springer-Nature Publishing
A breakdown in how brain cells rid themselves of waste precedes the buildup of debris-filled plaques known to occur in Alzheimer’s disease, a new study in mice shows.
For decades, scientists argued that such plaques, which contain the protein amyloid beta, built up outside of cells as a crucial first step toward the brain damage observed in Alzheimer’s disease. Led by researchers at NYU Grossman School of Medicine and the Nathan S. Kline Institute for Psychiatric Research, the new study challenges this idea, known as the amyloid cascade hypothesis.
The latest study findings argue instead that neuronal damage characteristic of Alzheimer’s disease takes root inside cells well before these thread-like amyloid plaques fully form and clump together in the brain.
Publishing as the cover article in the journal Nature Neuroscience online June 2, the study traced the root dysfunction observed in mice bred to develop Alzheimer’s disease to the brain cells’ lysosomes. These are small sacs inside every cell filled with acidic enzymes involved in the routine breakdown, removal, and recycling of metabolic waste from everyday cell reactions, as well as from disease. Lysosomes are also key, researchers note, to breaking down and disposing of a cell’s own parts when the cell naturally dies.
As part of the study, researchers tracked decreasing acid activity inside intact mouse cell lysosomes as the cells became injured as a result of the disease. Imaging tests developed at NYU Langone and the Nathan Kline Institute to track cellular waste removal showed that certain brain cell lysosomes became enlarged as they fused with so-called autophagic vacuoles filled with waste that had failed to be broken down. These autophagic vacuoles also contained earlier forms of amyloid beta.
In neurons most heavily damaged and destined for early death as a result, the vacuoles pooled together in “flower-like” patterns, bulging out from the cells’ outer membranes and massing around each cell’s nucleus. Accumulations of amyloid beta formed filaments inside the cell, another hallmark of Alzheimer’s disease. Indeed, researchers observed almost-fully formed plaques inside some damaged neurons.
“Our results for the first time sources neuronal damage observed in Alzheimer’s disease to problems inside brain cells’ lysosomes where amyloid beta first appears,” says study lead investigator Ju-Hyun Lee, PhD.
“Previously, the working hypothesis mostly attributed the damage observed in Alzheimer’s disease to what came after amyloid buildup outside of brain cells, not before and from within neurons,” says Dr. Lee, a research assistant professor in the Department of Psychiatry at NYU Langone and research scientist at the Nathan Kline Institute.
“This new evidence changes our fundamental understanding of how Alzheimer’s disease progresses; it also explains why so many experimental therapies designed to remove amyloid plaques have failed to stop disease progression because the brain cells are already crippled before the plaques fully form outside the cell,” says study senior investigator Ralph A. Nixon, MD, PhD.
“Our research suggests that future treatments should focus on reversing the lysosomal dysfunction and rebalancing acid levels inside the brain’s neurons,” says Dr. Nixon, a professor in the Departments of Psychiatry and Cell Biology at NYU Langone, as well as director of the Center for Dementia Research at the Nathan Kline Institute.
Researchers say they are already working on experimental therapies to treat the lysosomal problems observed in their studies.
A recent study published in April in Science Advances by the NYU Langone team sourced one cause of the cell’s waste disposal problems to a gene called PSEN1. The gene has long been known to cause Alzheimer’s disease, but its additional role in causing the illness through lysosomal dysfunction is only now becoming clear.
Their recent work also showed that the neuronal damage in a PSEN1 mouse model of Alzheimer’s disease could be reversed by restoring proper acid levels in lysosomes.
This work is covered by U.S. patent 9,265,735 that is directed to methods of treating Alzheimer's disease based on reversing lysosomal de-acidification, the underlying cause of waste buildup. The terms and conditions of the patent are being managed in accordance with the policies of the health system.
According to the National Institute on Aging, part of the National Institutes of Health (NIH), more than 6 million Americans, most of them age 65 or older, have dementia—a progressive loss of thinking, remembering, and reasoning—due to Alzheimer’s disease.
Funding for these studies was provided by NIH grants P01AG017617, P50AG025688, and R01AG062376.
Besides Dr. Lee and Dr. Nixon, other NYU Langone and Nathan Kline Institute study investigators involved in this research are Dun-Sheng Yang, Chris Goulbourne, Eunju Im, Philip Stavrides, Ann Pensalfini, Cynthia Bleiwas, Martin Berg, Chunfeng Huo, James Peddy, Monika Pawlik, Efrat Levy, and Mala V. Rao. Additional co-investigators are Han Chan and Cedric Bouchet-Marquis at Thermo-Fisher Scientific in Hillsboro, Oregon; and Mathias Staufenbiel at the University of Tubingen in Germany.
Media Inquiries
David March
Phone: 212-404-3528
david.march@nyulangone.org