Photo: Lester V. Bergman/Getty
A 35-year-old female was admitted via the emergency department (ED) at NYU Langone’s Tisch Hospital in August 2020 with one week of fever, nausea, abdominal pain, and diffuse bone pain. She had a history of systemic lupus erythematosus (SLE), secondary antiphospholipid syndrome (APS) with a triple-positive aPL antibody profile, HELLP syndrome, and chronic thrombocytopenia. In 2018, she had been hospitalized for pneumonia-triggered catastrophic APS (CAPS) manifested by pancytopenia, non-infectious cholangitis, and diffuse bone pain related to widespread osteonecrosis that responded to a treatment regimen including therapeutic anticoagulation, plasmapheresis, steroids, and mycophenolate mofetil.
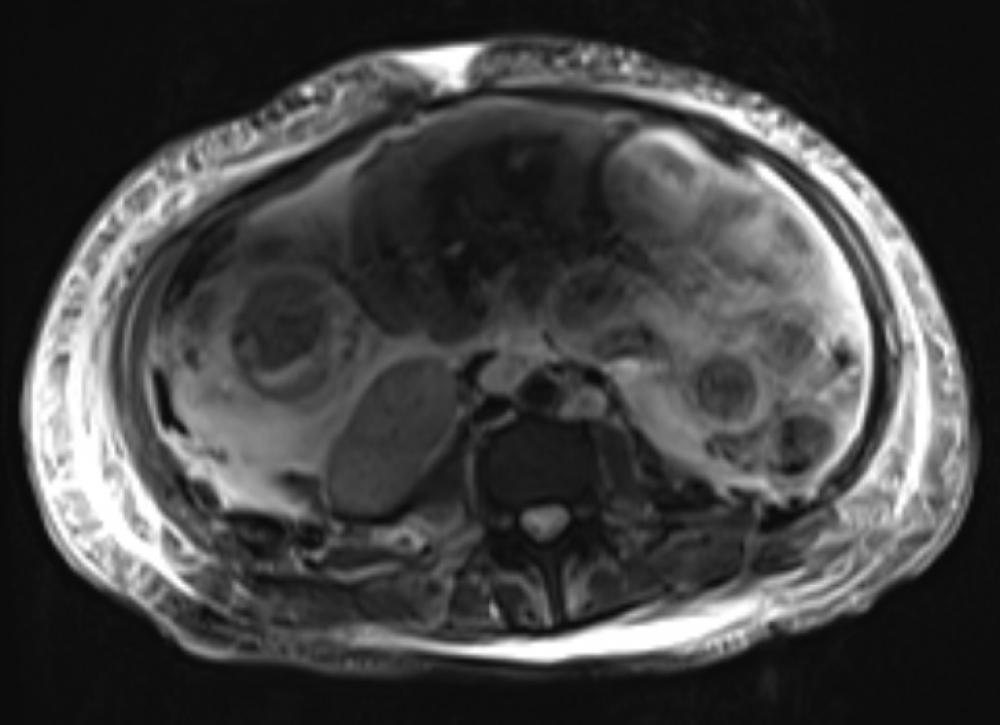
Tests in the ED demonstrated the presence of anemia, thrombocytopenia, mild peripheral schistocytes, and elevated liver enzymes. A CT scan revealed increased focal intrahepatic biliary ductal dilation. Magnetic resonance cholangiopancreatography (MRCP) showed a new focal biliary dilatation as well as multiple hypodense hepatic lesions, and MRI revealed a biliary stricture and likely portal vein thrombosis.
Given the patient’s medical history, elevated liver enzymes, hematological and imaging results, normal ADAMTS13 result, other lab and imaging results, fever, and intense chest and abdomen pain, H. Michael Belmont, MD, professor of medicine at NYU Langone, concluded that her presentation reflected a flare of atypical CAPS with widespread thrombosis-related ischemia with involvement in her bone, bone marrow, and hepatobiliary tract. Therapy was initiated with continuous heparin infusion, plasma exchange, and steroids. Due to the persistence of the patient’s fever, gastrointestinal symptoms of nausea, vomiting, and abdominal pain, as well as diffuse bone pain along with anemia and thrombocytopenia, cyclophosphamide and rituximab were added.
Ten days after her hospital admission, the patient developed acute bilateral blurry vision, followed by a nonoliguric acute kidney injury with nephrotic range proteinuria. In consultation with ophthalmology, Dr. Belmont diagnosed atraumatic Purtscher’s retinopathy and considered SLE, CAPS, and now diffuse microvascular injury involving the chorioretina and glomerular microvasculature as seen in complement-mediated thrombotic microangiopathic anemia. Based on this concern, Dr. Belmont recommended treatment with eculizumab, a monoclonal antibody inhibitor that prevents the generation of both C5a and C5b-9 complement components. He and colleagues also ordered atypical hemolytic uremic syndrome (aHUS) genetic testing, which revealed genetic variants in both the CFB and MCP (CD46) genes for complement regulatory proteins. “This patient was highly complex, with her lupus and secondary antiphospholipid syndrome unmasking an underlying genetic defect that also gave her complement-mediated HUS,” Dr. Belmont says.
Her condition continued to deteriorate, and she was transferred to the medical ICU for management including intravenous nicardipine for accelerated hypertension. In the ICU, the patient developed acute hematemesis and hematochezia with severe acute blood loss anemia requiring multiple transfusions. An MRA demonstrated prominent mural edema in her jejunum, consistent with an infectious, inflammatory, or ischemic enteritis. Stool cultures remained negative, consistent with an etiology of gastrointestinal bleed secondary to microvascular thrombosis from her thrombotic microangiographic hemolytic anemia (TMHA).
In the ICU, the patient continued to receive anticoagulation, plasma exchange, steroids, and additional doses of eculizumab as well as supportive care. Over time, her visual loss was restored; the gastrointestinal issues of nausea, pain, bleeding and elevated liver tests resolved; and her significant bicytopenia with anemia and thrombocytopenia and narcotic-requiring diffuse bone pain were resolved as well. Her acute kidney injury also improved, with peak creatinine of 3.6 mg/dL and urine protein excretion of 16,078 mg/g (creatinine) falling with successful treatment to 0.96 mg/dL and 654 mg/g (creatinine), respectively.
Due to her marked improvement, the patient was discharged from the hospital one month after her admission. This highly complex case, Dr. Belmont says, demonstrates the clear advantages of NYU Langone’s expertise in rheumatology, hematology, ophthalmology, nephrology, and intensive care. The close and frequent consultations allowed the interdisciplinary care team to quickly respond to an evolving and cascading suite of symptoms. Likewise, those consultations were crucial in resolving the patient’s refractory CAPS complicated by bone, bone marrow, liver, biliary, choroid/retina, kidney, and jejunum involvement in a patient with long-standing SLE, secondary APS, and underlying inherited polymorphisms in both her CFB and MCP genes.