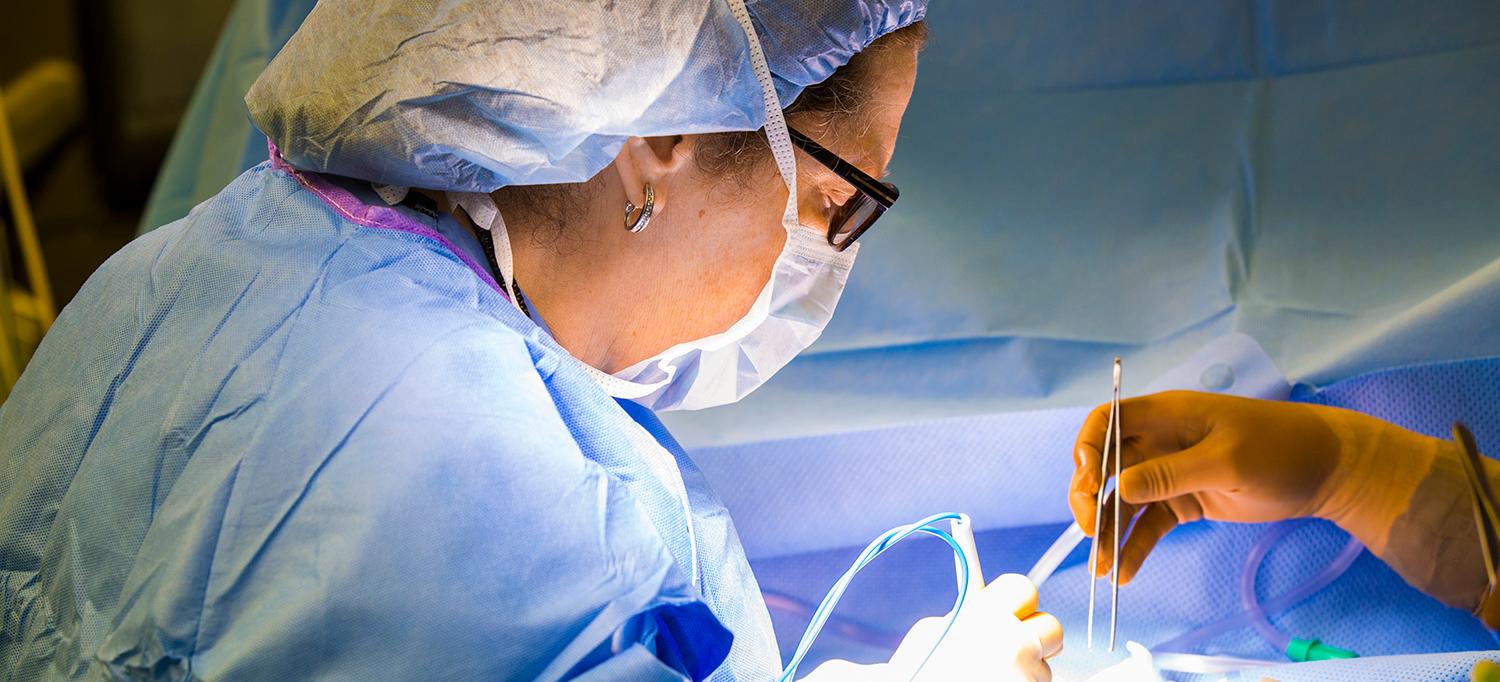
Dr. Freya Schnabel performs breast surgery.
Photo: Adam Watt
Breast surgeons at NYU Langone’s Perlmutter Cancer Center are taking major steps to improve outcomes for their patients. Using a device called MarginProbe, doctors can scan the outer edges of excised tissue to ensure they are free and clear of any suspicious breast cancer cells. This helps NYU Langone and other institutions across the globe eliminate the need for follow-up procedures—an often worrisome part of breast cancer treatment.
Freya Schnabel, MD, director of breast surgery at Perlmutter Cancer Center, conducted the original clinical trials on MarginProbe that led to its FDA approval. “MarginProbe allows us to increase the likelihood that patients will leave the OR with a successful lumpectomy,” Dr. Schnabel tells The Wall Street Journal. “This technology is a real advance, and represents a further refinement of the lumpectomy procedure.”
MarginProbe is a wand-like device about the size of an electric toothbrush. The surgeon runs the device around the edges of excised tissue, and the attached monitor then indicates to the surgeon if the margins are clean or if there are still cancer cells present. If cells remain, the surgeon removes additional tissue while the patient is still on the operating table—all but eliminating the need for additional surgery.
Read more from The Wall Street Journal (subscription required).

