Image: wildpixel/Getty
A newfound feature of human anatomy made up of a series of compartments is interconnected throughout the body, researchers at NYU Grossman School of Medicine have discovered. This anatomical “highway” provides a route for normal signals and may potentially be involved with spreading cancer cells.
This is the finding of a study published online March 31 in Communications Biology, an open access journal of Nature. It builds on work published in 2018, in which pathologists from NYU Grossman School of Medicine revealed that widespread layers of the body thought to be solid connective tissues instead contained fluid-filled spaces, comprising a previously unknown, body-wide system called the interstitium.
The 2018 study suggested that the natural movements of the body—the pulsing of heart and blood vessels, the rhythmic squeezing that moves food through the digestive tract, the flexing of muscles—push fluid through the network, supported by a mesh of connective tissue proteins, creating the possibility that it carries signals.
What remained to be proven was whether these compartments—found below the skin’s surface, and lining organs, arteries and veins, nerves, and fascia between muscles—are separate units confined to their local tissues or are interconnected throughout the body and across organ boundaries.
“Our experiments suggest that interstitial spaces are continuous, creating a potential superhighway through the body, distinct from blood and lymphatic vessels, though which cells and molecules circulate,” says lead study author Odise Cenaj, MD, assistant professor in the Department of Pathology at NYU Langone Health. “This finding may have significant implications for how the body signals to itself across long distances to maintain health, as well as for the spread of certain cancers.”
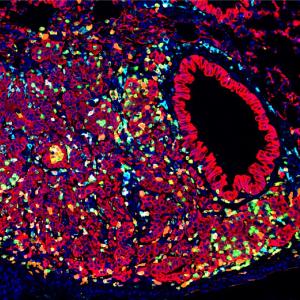
“Clear imaging of this network of spaces is difficult with standard radiologic imaging techniques,” says senior study author, Neil D. Theise, MD, professor in the Department of Pathology at NYU Langone. “To study the question of continuity, we had to find new ways to map them.”
Tattoos and Silver
The team first sought to reveal interstitial connections by tracking movement through them using non-biological particles—of tattoo ink and silver—from the point where they were first deposited. In tissue samples taken from skin biopsies, injected tattoo ink particles and silver particles applied on the skin surface were observed to move via interstitial spaces through all skin layers and then further, through the connective tissue layer underneath them (the fascia). The very small silver particles also moved into the connective tissue surrounding nerves and blood vessels, thereby potentially passing along to distant parts of the body.
Tattoo ink is also used to mark some cancerous colon polyps before surgical removal, a standard procedure that let the team examine pigment movement there as well. As in the skin, the ink particles deposited in the colon’s inner layers travelled through all deeper layers of the bowel wall, and out into the connective tissue of the mesentery beyond.
A third set of experiments exploited a naturally occurring substance called hyaluronic acid (HA), a large molecule known to hold water and contribute to fluid balance. Interstitial spaces appear white (empty) in anatomical studies using standard stains, but when the research team stained HA brown, they found the gel-like substance to occupy every interstitial space. Observing no gaps in the HA throughout all interstitial spaces, the authors concluded that such spaces are continuous, with open channels running through all connective tissues.
Dr. Theise says the results may have implications for questions surrounding the growth of certain cancers, including some in the skin (in-transit melanomas) and colon (mesenteric colon cancer implants distinct from the main tumor). These cancers originate in one spot and then spread to another distant location despite having no clear anatomical pathway (e.g., nerve, artery, or lymph vessel) to follow. In their tissue slides, the study authors saw evidence suggesting that cancer cells could be spreading from their sites of origin through the local interstitium, but further studies will be needed to confirm this theory.
Some cancer cells emit enzymes that eat through the surrounding tissue to make way for invasive growth, say the authors. However, research in the field has shown that early on, such invasion often proceeds without tissue breakdown by enzymes, and could happen instead through pre-existing spaces.
“Our study supports this concept, and now we are mapping those spaces,” says Dr. Theise. “Again, more work will be needed to determine the mechanisms by which cancer cells may migrate through interstitium.”
Moving forward, the team is also developing techniques to examine the protein content in interstitial spaces to understand whether or not nearby tumors alter interstitial spaces to enable cancer cell movement through them. Their goals are to determine if signals predict whether a certain cancer type, such as melanoma, is likely to spread through the interstitium, and if so, to find ways to block it.
Along with Dr. Cenaj and Dr. Theise, authors of the study from the Department of Pathology at NYU Langone are Douglas Allison, Rami Iman, Briana Zeck, Lilly Drohan, Luis A. Chiriboga, and Cheng Z. Liu. Also authors are Jessica Llewellyn and Rebecca Wells of the Perelman School of Medicine at the University of Pennsylvania; and Young Nyun Park in the Department of Pathology at Yonsei University College of Medicine in Seoul, South Korea. The study was funded by a Perlmutter Cancer Center support grant, and by National Institutes of Health grants P30CA016087, S10OD01058, and S10018338.
Media Inquiries
Greg Williams
Phone: 212-404-3500
gregory.williams@nyulangone.org