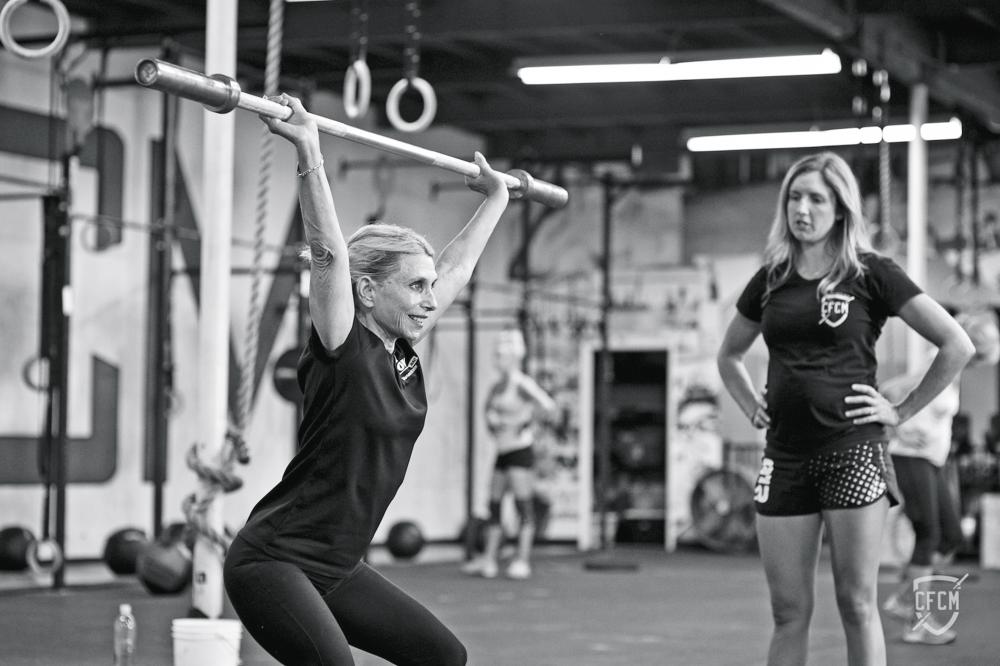Nancy Clayton, 73, just a few months after receiving an experimental mitral valve.
Photo: Michael Grecco
At 10:00 on a bright August morning, Nancy Clayton met her rowing coach at a marina in Newport Beach, California. The two exchanged a long hug. “I can’t believe you’re back,” exclaimed the coach, a fit young woman named Jill Clapp. “I can’t, either,” Clayton said.
Together, they carried Clayton’s single-scull racing boat to the dock and slid it into the water. Clayton climbed aboard the low-slung craft, moving carefully to keep the narrow hull from tipping over. Then, she pushed off into the bay, her legs pumping and her seat sliding back and forth with each sweep of her oars. Clapp followed in a motor launch.
Other boaters out that day might have been shocked to learn that the rower skimming past them—with her stylish blond bob and sleek athletic togs—was 73 years old. But there was something even more astonishing about Clayton: just five weeks earlier, doctors had replaced a diseased valve in her heart.
As she paddled, she recalled her two previous valve procedures, open-heart surgeries that required months of grueling recovery. This time, she’d traveled across the country to NYU Langone Medical Center, where she’d volunteered for a groundbreaking experiment: a mitral valve replacement involving no surgery at all. Now, here she was, propelling herself through a light chop, pelicans wheeling overhead, and so far, she’d barely broken a sweat.
VIDEO: Nancy Clayton came to the Heart Valve Center to receive one of the first transcatheter mitral valve replacements.
A few hundred yards offshore, Clapp pulled alongside. “Ready for some drills?”
Clayton felt a surge of anxiety. This would be her first major workout since the operation. How would her newly mended heart behave? There was only one way to find out. “Let’s go,” she said.
Nancy Clayton hadn’t planned to make medical history in January 2016, when she phoned Aubrey Galloway, MD, the Seymour Cohn Professor of Cardiothoracic Surgery and chair of the department. All she wanted was a second opinion from a physician who years earlier had amply earned her trust.
Born with a malformed aortic valve, Clayton had been defying the odds for seven decades. As a girl, she’d ignored her worried mother’s ban on exercise and become an avid fitness buff. After valve-repair surgery in her early 30s, she went back to the gym as soon as she was able. When the repair gave out, she was 58 years old and living in New York City. She needed a mechanical replacement valve and a pacemaker, along with a procedure to enlarge her aortic root so that the new valve would fit. Clayton’s husband, Zane Miranda, MD, a dermatologist who’d done his residency at NYU Langone, had heard from a colleague that Dr. Galloway was a leader in his field.
In a dramatic twist, the operation took place on September 11, 2001, as the towers collapsed five miles downtown. Dr. Galloway had just connected Clayton to a heart–lung machine and was sawing open her chest when the second plane hit. Every few minutes someone rushed into the operating room with an update. “Please keep the door shut,” Dr. Galloway said sternly. “What’s happening is horrible, but we can’t do anything about it right now. We’ve got to take care of our patient.”
Fourteen years later, after following her son and two grandchildren to Southern California, Clayton was in the best shape of her life. Most days, the nonprofit marketing specialist spent an hour practicing with her competitive rowing team or an hour doing CrossFit training—or both. But one morning in late 2015, she felt unusually winded as she labored at the oars. Over the following weeks, her shortness of breath worsened, and she developed a chronic cough.
When Clayton’s doctor ruled out bronchitis, she went to see her cardiologist. The tests came back with distressing news: this time, the problem wasn’t with her aortic valve. Instead, she had mitral valve regurgitation—a disorder in which the valve fails to close completely, causing some blood to flow backward into the heart’s upper chamber. Eventually, the condition can lead to congestive heart failure. Clayton had begun to reach that stage.
The question was how to treat it. For an elderly patient who’d already undergone two open-heart surgeries, a third could be debilitating, even fatal. But while far gentler alternatives had been developed for the aortic valve, such options for its mitral counterpart were severely limited.
So she called Dr. Galloway. Soon afterward, Clayton returned to NYU Langone for another brush with history—this one more auspicious.
The mitral valve serves as the heart’s gatekeeper, regulating the flow of oxygenated blood from the left atrium into the left ventricle. Because it handles higher pressure levels than the other three cardiac valves, it often gives out first. At its top, a fibrous ring called the annulus supports two leaflets, whose motion is controlled by cordlike tendons. Mitral valve regurgitation (MR) occurs when the leaflets don’t meet properly, allowing blood to flow backwards.
MR is the most common form of valve disease, affecting an estimated 4 million Americans. The type Clayton had—known as degenerative MR—often results from simple wear and tear. Although mild cases can often be controlled with medication, the only effective treatment for severe MR may be to repair or replace the errant valve. Yet of the 1.7 million patients who become eligible for mitral-valve surgery each year, only 30,000 undergo it.
One important reason for this, many studies suggest, is that MR is strongly associated with advancing age—and older people tend to be poor candidates for the rigors of open-heart surgery. Some are too frail to survive such a procedure; others may be unable or unwilling to weather the months of painful recovery that follow. “These surgeries work very well, but they’re not for everybody,” says Mathew R. Williams, MD, associate professor of cardiothoracic surgery and medicine, and director of the Heart Valve Center.
Over the past two decades, researchers have developed an array of techniques aimed at reducing the impact of valve repair or replacement procedures on patients’ bodies. Such methods are usually reserved at first for those at high risk of dying from surgery, but they may be approved for a broader population as technologies are refined and data on safety and efficacy accrue. One of the most widely used forms of minimally invasive cardiac surgery was pioneered in the 1990s by Dr. Galloway. Known as the minithoracotomy, it provides access to the heart between the ribs instead of through the breastbone—shrinking the incision, decreasing the trauma, and lessening the likelihood of complications. But even that approach, which still requires stopping the heart and using a heart-lung bypass machine, may be too taxing for many patients.
More recently, advances in imaging and other technologies have enabled a growing number of cardiovascular procedures to be performed percutaneously—that is, through a skin puncture rather than an incision. The best-known example may be transcatheter aortic valve replacement (TAVR), which has given a new chance at life to as many as 50,000 desperately ill patients in the U.S., and thousands more abroad. Guided by live imaging, an interventional cardiologist typically inserts a catheter through a puncture in the groin, snakes it through the femoral artery, pushes the old valve out of the way, and seats the substitute in its place. Because the heart continues to beat, no heart-lung machine is necessary. The FDA approved TAVR for inoperable patients in 2011, for high-risk patients in 2012, and for moderate-risk patients in August of this year. Trials are under way for the low-risk cohort. About 30 percent of aortic valve replacements are now performed by this method.
“TAVR has changed the standard of care for aortic valve disease in older patients,” Dr. Galloway observes. “If we can do something similar for mitral valve disease, it will be transformative.”
But it won’t be easy. The mitral valve presents a gauntlet of obstacles. Unlike the aortic valve, it can’t be accessed directly through an artery; instead, the catheter must penetrate the wall of the heart, creating major technical challenges for device designers and operators. The aortic valve, moreover—with its round annulus at the end of a gently curving tube—is a much simpler structure. The mitral valve’s annulus is large and saddle-shaped, and contracts each time the leaflets close. The atrium above it is a tight space, with little room to maneuver.
That’s why progress on transcatheter treatments for mitral valve disease has been comparatively slow. In this country, the sole device available for percutaneous repair of MR is the MitraClip, approved by the FDA in 2013 for high-risk patients with the degenerative form of the disease. The clip works by gripping the edge of the leaflets, pulling them closer together. But while it reliably reduces severe regurgitation to a more manageable level, it seldom eliminates the condition completely.
For patients like Nancy Clayton, who would benefit from mitral valve replacement rather than repair, the transcatheter option is available only through a clinical trial. Dr. Williams, who has performed nearly 3,000 TAVRs, more than any other U.S. surgeon, is one of just a handful of surgeons worldwide authorized to conduct such studies.
When Clayton contacted Dr. Galloway early this year, he suggested that she might be a candidate for a MitraClip instead of surgery, and referred her to Dr. Williams. After examining her test results, Dr. Williams told Clayton, “We may have something else for you.”
Clayton flew to New York a week later. Dr. Williams explained that there were three possible alternatives for treatment. The first was the MitraClip, which could stabilize her condition but might not enable her to return to the active way of life she cherished. The second choice was open-heart surgery. Dr. Williams, the first physician in the U.S. trained in both interventional cardiology and cardiac surgery, felt confident that he and his colleagues could safely replace her mitral valve by this method, as they had done for many other high-risk patients. But he warned that she was at elevated risk for complications largely due to her prior surgeries, and her recovery would likely be even more challenging than it was 15 years earlier, when she endured six weeks of chest pain and exhaustion.
Then came the third choice: transcatheter mitral valve replacement, or TMVR. About two dozen manufacturers were developing such devices, Dr. Williams said, and a few had begun human trials. The results so far had been less than stellar, with 30-day mortality rates ranging from 25 percent to 50 percent. In part, this reflected the advanced age and severe illness of most trial participants; many died of causes unrelated to their valve replacement soon after the procedure. But in other cases, the devices themselves may have contributed to the problem. All of the implants were designed to be delivered through the apex of the heart. This is the most direct route to the mitral valve, but it requires entering through an incision in the chest and placing stitches in the apex, both of which are physiologically stressful.
Dr. Williams, however, had been selected to test a new device that took a fully percutaneous approach. As with the MitraClip, the catheter was inserted through the groin and up the femoral vein, then pushed through a tiny puncture in the atrial septum—the inner wall that separates the upper right and left sides of the heart. Although more difficult for clinicians, transseptal delivery was far easier on patients.
The new device had other potential advantages, as well. Unlike other TMVR systems, it was expressly designed for the mitral valve, rather than repurposed from an aortic device. An anchoring ring was installed separately from the valve itself, which made for less bulk. Each part could be withdrawn and repositioned repeatedly for a better fit. “With other devices,” Dr. Williams explained, “once you’ve placed it, you’re done. There’s no retrievability.”
Thanks to recent changes in FDA policy, he added, the artificial mitral valve would be the first TMVR device to begin early feasibility studies in the U.S. rather than Europe or elsewhere in the world, and Clayton could become the first human subject to receive the implant. She asked for some time to deliberate, and flew back to California.
“I was up every night, stressed to the max,” Clayton recalls. The conventional options were unappealing, she thought, but their risks were known. With TMVR, Dr. Williams had cautioned her, the potential complications were similar—stroke, fluid buildup around the heart, or dying during the procedure—but no one could gauge the odds.
Clayton’s family rallied to help with the decision. After speaking with Dr. Williams and Dr. Galloway, her son, a veterinary cardiologist, thought TMVR was the right decision. Her husband agreed. But it was her own research that finally convinced her. “I googled Dr. Williams,” she recalls, “and people were calling him a genius.” She told him she was willing—though she preferred to be the second subject, not the first.
A woman in her 80s preceded her, on June 15. The procedure went perfectly.
Clayton’s turn came on July 7. At 8:30 a.m., she was wheeled into NYU Langone’s newest state-of-the-art hybrid OR (one of two at the Medical Center equipped for both cardiac surgery and interventional cardiology) and placed under general anesthesia. More than a dozen clinicians joined Dr. Williams around the movable operating table, which was encircled by a C-arm fluoroscope. A bank of wide-screen monitors glowed overhead. With Clayton mostly hidden under surgical drapes, the procedure that followed looked more like a video game than surgery, albeit one with a real life at stake.
As Muhamed Saric, MD, PhD, associate professor of medicine and medical director of noninvasive cardiology, inserted a special camera scope down Clayton’s throat, a video of her mitral valve appeared onscreen—the regurgitation clearly visible. After inspecting the anatomy on display, Dr. Williams pushed a catheter roughly the width of a permanent marker into Clayton’s right groin, using a control handle studded with dials and switches to slowly thread it up the vein. He used other controls to continually reposition the table and C-arm, enabling the X-ray camera to follow the action. On the monitors, the catheter appeared as a sinuous gray form, silhouetted against a tracery of blood vessels.
When the tip of the catheter reached the heart, the full-color, computer-enhanced echocardiography became crucial. Dr. Saric kept up a constant dialogue with Dr. Williams, changing the angle and focus of the echo camera as needed to ensure millimeter-scale accuracy. Entering the right atrium, Dr. Williams used a retractable needle to pierce the septum. He advanced into the left atrium, steering sharply downward toward the mitral valve. Then, he loaded the two-stage device—its components rolled like umbrellas—into the catheter.
The anchoring unit emerged first, slipping down into the diseased valve. As the unit unfurled, a network of metal-alloy braces spread across the underside of the annulus. On top of the annulus, a cloverleaf-shaped receptacle unfolded. Using a tiny grasping tool, Dr. Williams positioned the anchor for maximum stability and locked it into place. Finally, he deployed the replacement valve (a metal frame with three porcine-tissue leaflets sewn into the middle) and attached it to the anchor. In an instant, Clayton’s new mitral valve was opening and closing smartly with each heartbeat.
At 12:30 p.m., Dr. Williams called out, “That’s it! Thanks, everyone.” As Clayton was rolled out to the recovery room, the sense of relief was palpable. She had survived the operation. But no one could say what would come next.
Sadly, the first patient to receive the experimental mitral valve died a month after the procedure, of noncardiac problems that the device was not meant to solve. Clayton, however, was up and walking less than two hours after the procedure. She was discharged from the hospital four days later (she could have gone home after just one day if her blood thinners hadn’t needed adjusting), and she was back on the treadmill the next morning. Within two weeks, she was walking two miles a day. Three weeks after that, she returned to Newport Bay.
Clayton ran through a series of exercises emphasizing the upper or lower body, as Coach Clapp shouted, “Square your blade! Straighten your back!” She tried to pay attention to her form, but waves of joy kept distracting her. Although she had to stop and catch her breath every 10 minutes or so, she managed to keep going for a half-hour before Clapp suggested they head back to shore. “How did that feel?” the coach asked as they stepped onto the dock. Clayton just grinned.
Today, she has resumed her old exercise schedule, functioning at what she gauges to be 80% of her potential capacity. “I’m getting stronger every week,” Clayton reports, “and I absolutely expect to get back to where I was before, and then some. It feels like a miracle.”
That miracle was made possible by techniques that could hardly have been imagined until recently. “If you’d told me 10 years ago that we could replace mitral valves percutaneously, I would have said, ‘You’re crazy,’ ” Dr. Saric says with a laugh.
Yet such wonders aren’t likely to remain rare for long. “These devices are in their infancy, and we still need to see which ones will work best,” says Dr. Williams. “But this type of technology represents the future. Someday, I hope we can offer it to everyone.”