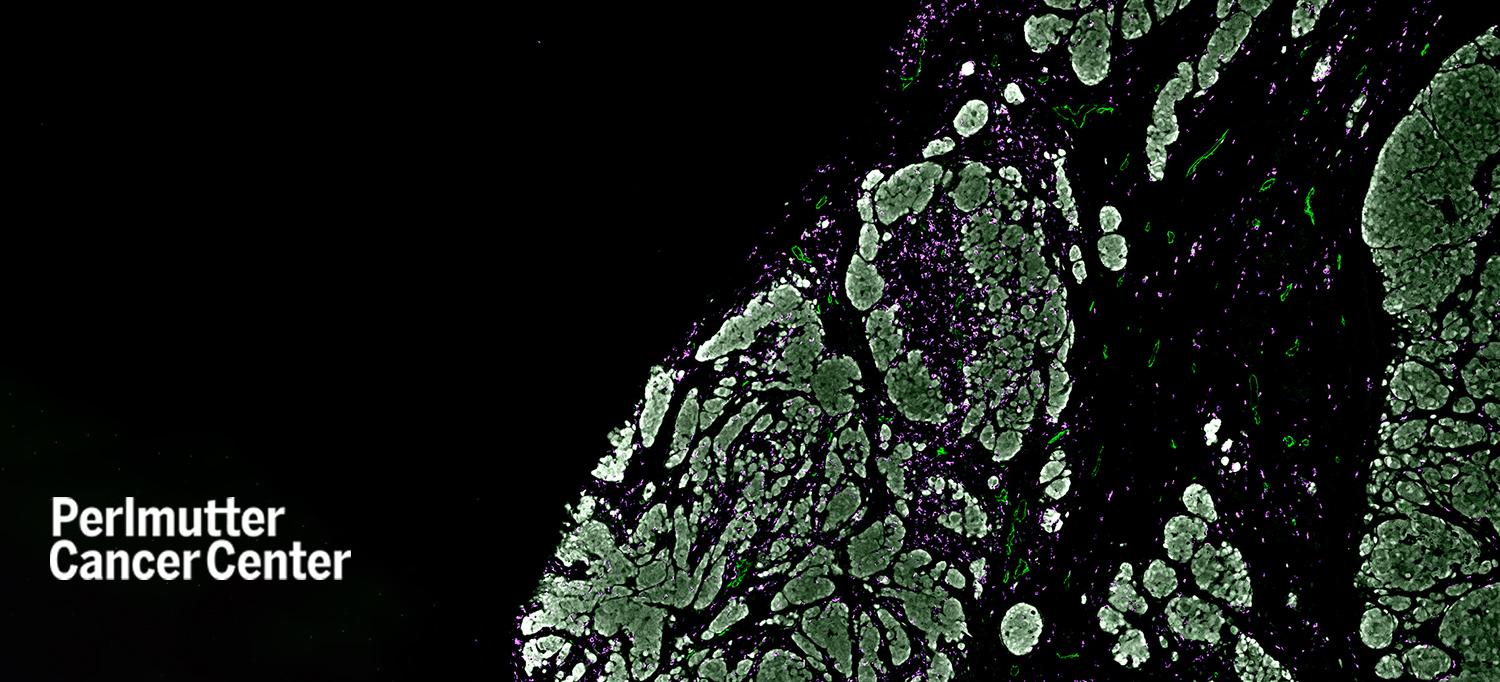
Immune T cells (purple) excluded from skin tumors, or melanoma (white), leak out through lymphatic vessels (green).
Image Courtesy of Ronald O. Perelman Department of Dermatology
The number of specialized immune cells available for fighting skin cancer doubled when a new treatment blocked their escape from melanoma tumors, experiments in mice and human cells show.
Researchers at NYU Langone Health and its Perlmutter Cancer Center who led the study found that combining a chemical blocker of immune cell exit with another drug type, an immunotherapy, stopped melanoma tumor enlargement in more than half of mice tested. Immunotherapy alone had previously failed to prevent the cancer’s growth.
Recent advances in immunotherapies, medications designed to help the body’s immune defense system detect and kill cancer cells, much like it would an invading virus, have greatly improved cancer care, researchers say. The treatments work by boosting the action of immune cells that both directly attack the cancer and prevent cancer cells from evading recognition by the immune system.
The latest generation of immunotherapies, called immune checkpoint inhibitors, protect antitumor T cells from inactivation and have become a mainstay in the treatment of melanoma. While these drug treatments do not work for all patients, previous research shows that having more overall T cells, particularly when positioned in the center of tumors, makes the drugs more effective.
The new study, published online February 27 in the journal Nature Immunology, showed that key immune cells called CD8 T cells escape melanoma tumors when they gather near the tumor periphery as well as nearby lymphatic vessels, which carry immune cells throughout the body. Indeed, the researchers found that more T cells accumulate inside tumors in mice bred to lack lymphatic vessels in their skin.
Further experiments showed that signaling molecules, chemokine CXCL12 and its related receptor protein CXCR4, attract and move T cells toward lymphatic vessels. When researchers blocked either CXCL12 or CXCR4, T cells could not emigrate from the tumor and instead stayed in its center.
Taken together, the researchers say the results demonstrate how T cells are likely drawn to the tumor’s outer rim by CXCL12 and closer to the lymphatic vessels, where CXCR4 “encourages” the T cells to exit the tumor. When researchers combined immunotherapy with a chemical blocker of CXCR4, the number of T cells in mice tumors doubled and half of tumors stopped growing.
“Our study confirms for the first time how CD8 T cells are escaping melanoma tumors through chemokine signaling to their nearby lymphatic vessels, leaving tumors less susceptible to anticancer immunotherapy,” said study lead investigator Maria Steele, PhD, a postdoctoral research fellow in the Ronald O. Perelman Department of Dermatology at NYU Grossman School of Medicine and Perlmutter Cancer Center. “These findings reveal that T cells circulate out of tumors, reshaping scientific views of tumor immunology where T cells randomly find and target tumor cells.”
“Our study shows that blocking this escape route lets immunotherapy work better in fighting the growth of skin cancer cells,” said study senior investigator Amanda W. Lund, PhD.
Among the study’s other results was that T cell leakage depended on their potency, or how strongly they could bind to target proteins on tumor cells. The longer the most potent T cells spent inside tumors, the more likely they were to encounter their target cancer cells and the more likely these T cells were to remain inside the tumor. Increasing the initial time these T cells spend inside the tumor, the researchers say, may help improve therapy.
“These results suggest that it is not only about getting T cells into the melanoma tumor but also about getting these T cells to the right place with the right signals to drive the most specific and durable immune responses,” said Dr. Lund, an associate professor in the Ronald O. Perelman Department of Dermatology and Department of Pathology at NYU Grossman School of Medicine and a member of Perlmutter Cancer Center.
Researchers say the study overall shows that the lymphatic system likely recirculates T cells out of tumors. And that for some patients, blocking the exit signals—CXCR4 and/or CXCL12—is needed to shift the balance in favor of keeping T cells inside of tumors long enough for immunotherapy to work.
Future research will look at how blocking tumor T cell exit influences immunotherapy, say the authors, who also plan to look at targeting not just the chemokine signals but also other molecular pathways in the lymphatic vessels to increase the “dwell time” of T cells inside tumors.
Funding for the study was provided by National Institutes of Health grants P30CA016087, P30CA069533, P30CA0695533, R01CA238163, T32CA106195, and T32GM136542; the Cancer Research Institute; the Melanoma Research Alliance; Stand Up to Cancer—Lustgarten Foundation Pancreatic Cancer Convergence Dream Team Translational Research Grant SU2C-AACR-DT14-14; and the Swedish Research Council. Dr. Lund is a paid consultant for AGS Therapeutics (AGS Tx), a biopharmaceutical company based in Paris, France, that was not involved in the study. The terms and conditions of this arrangement are being managed in accordance with the policies of NYU Langone Health.
Besides Dr. Steele and Dr. Lund, other NYU Langone researchers involved in this study are Ian Dryg, Haley du Bois, and Cameron Hill. Other study co-investigators are Dhaarini Murugan, Julia Femel, Sancy Leachman, Young Chang, and Lisa Coussens, all at the Oregon Health & Science University in Portland.
Media Inquiries
David March
Phone: 212-404-3528
david.march@nyulangone.org