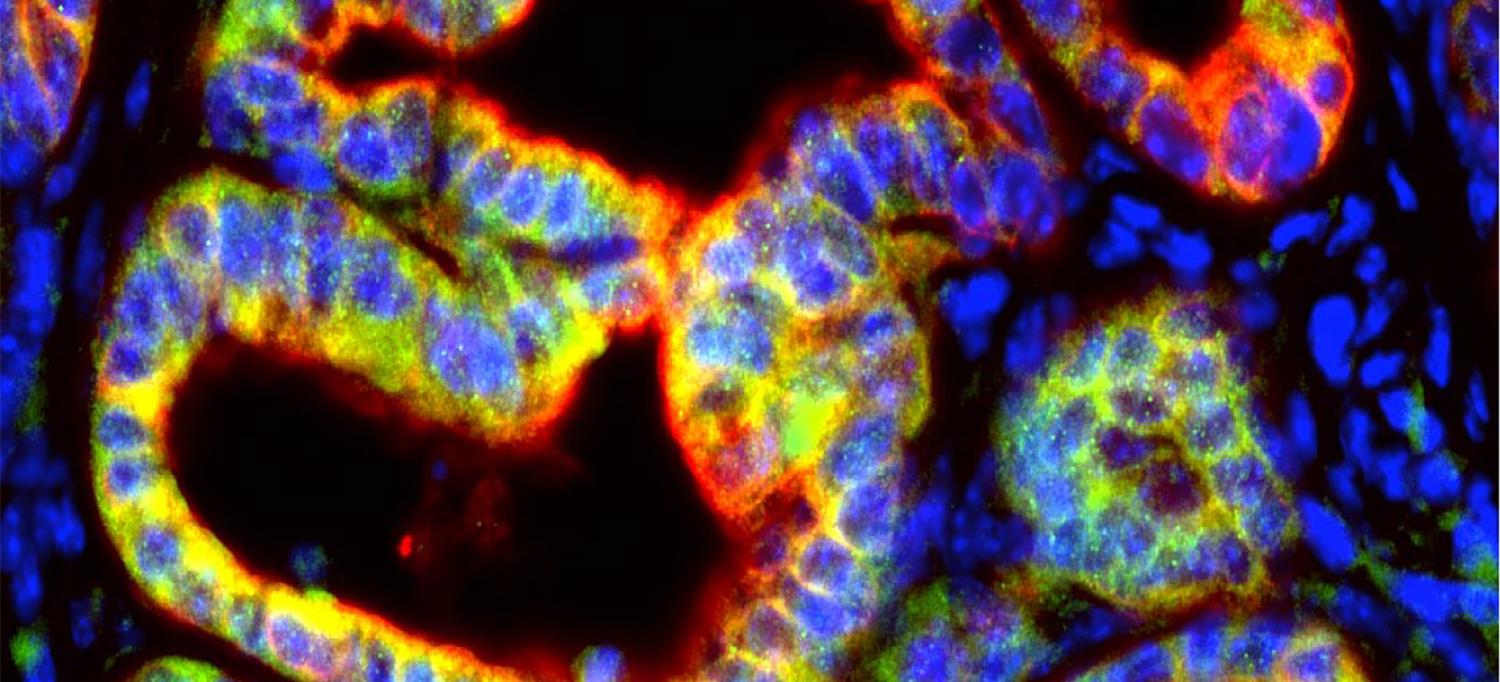
Mouse pancreatic tumor cells, in red, produce the signaling protein IL-1β, in green, to support cancer growth by suppressing the immune system.
Image Courtesy of NYU Grossman School of Medicine
A key immune signal has a previously unknown role in turning off the immune system’s attack on pancreatic cancer cells, a new study finds.
Led by researchers at NYU Grossman School of Medicine, the study found that an immune signaling protein, interleukin-1β (IL-1β), is made and released by pancreatic tumor cells. This was shown to reduce anti-cancer immune responses, which promoted the growth of pancreatic ductal adenocarcinoma, or PDA, a form of cancer that is usually deadly within two years.
Published online in Cancer Research, a journal of the American Association for Cancer Research, on January 8, the study found that blocking the action of IL-1β in mice with immune proteins called antibodies caused a 32 percent decrease in PDA tumor growth.
Other experiments combined the anti-IL-1β antibody, which gloms onto and neutralizes its target, with an already approved antibody treatment that shuts down the protein “checkpoint” called PD-1. To spare normal cells from immune attack, the immune system uses “checkpoints” on immune cells that turn them off when they receive the right signal. Cancer cells hijack checkpoints to turn off the system, leading to immune suppression of CD8+ T cells that would otherwise kill cancer cells. Therapies called checkpoint inhibitors counter this effect.
Though effective against many cancers, checkpoint inhibitors have failed in pancreatic cancer, with the response rate in tumors as low as roughly three percent in some trials, and the limitations attributed to poor CD8+ T cell infiltration and immune suppression. In the current study, adding anti-IL-1β antibodies to anti-PD-1 antibody treatment doubled the infiltration of such T cells into PDA tumors and increased the anti-tumor activity of PD-1 blockade by 40 percent.
“By engineering mice with versions of PDA that lack the IL-1β gene, we found for the first time that pancreatic cancer cells produce IL-1β, and that it is essential for the continued growth of PDA tumors,” says study senior author Dafna Bar-Sagi, PhD, executive vice president and vice dean for science, and chief scientific officer for NYU Langone Health. “Blocking IL-1β with an antibody treatment may represent another way to make pancreatic tumors vulnerable to the immune system, with the potential to significantly increase the effectiveness of checkpoint inhibitors if combined.”
Initial Trigger
The new finding is in line with past work in other labs that had described the microbiome, the mix of bacterial species in the pancreas, as being altered in the presence of PDA, and a factor in cancer growth. The field had traditionally assigned the production of IL-1β to immune cells, but the new work finds that pancreatic tumor cells can also make it in response to proteins given off by certain bacteria.
Bacterial products were found to activate proteins on the cancer cell surfaces called toll-like receptors, which set off chain reactions that were required for IL-1β production in cancer cells.
The research team also found that higher IL-1β production caused nearby pancreatic stellate cells to increase production of dense, structural proteins such as collagen. Desmoplasia is the overgrowth of such fibrous tissue that often occurs near pancreatic tumors, and which has been linked to treatment resistance.
Active stellate cells were also found to trigger production of the signaling proteins that attract immune cells called macrophages into tumors and programs them to become the type (M2) that suppresses immune reactions. Experiments also confirmed that higher IL-1β and M2 macrophage levels, along with fibroblast-driven desmoplasia, reduced the ability of cancer-cell-killing CD8+ T cells to enter tumors.
“This work provides strong evidence that blocking the action of IL-1β enables T cells to better penetrate tumors and kill cancer cells, mechanisms with potential to overcome the limitation of currently available immunotherapies in the treatment of pancreatic cancer,” says first author Shipra Das, who was a member of Dr. Bar-Sagi’s lab at the time the study was done.
Along with Dr. Bar-Sagi and Das, study authors from the Department of Biochemistry and Molecular Pharmacology at NYU Grossman School of Medicine were Beny Shapiro, Emily Vucic, and Sandra Vogt. Das, who was a postdoctoral fellow, has since left NYU Langone and continues her work in immune oncology in the biopharmaceutical industry.
While no study author received financial compensation, the pharmaceutical company Novartis provided the mouse anti-PD1 and anti-IL-1β antibodies for this study. The work was supported by National Institutes of Health grants P30CA016087, CA210263, and T32GM115313, as well as by support from the Lustgarten Foundation Pancreatic Cancer Convergence Dream Team grant SU2C-AACR-DT14-14, Stand Up To Cancer, the Entertainment Industry Foundation, American Association for Cancer Research, and the Canadian Institutes of Health Research.
Media Inquiries
Greg Williams
Phone: 212-404-3500
gregory.williams@nyulangone.org

