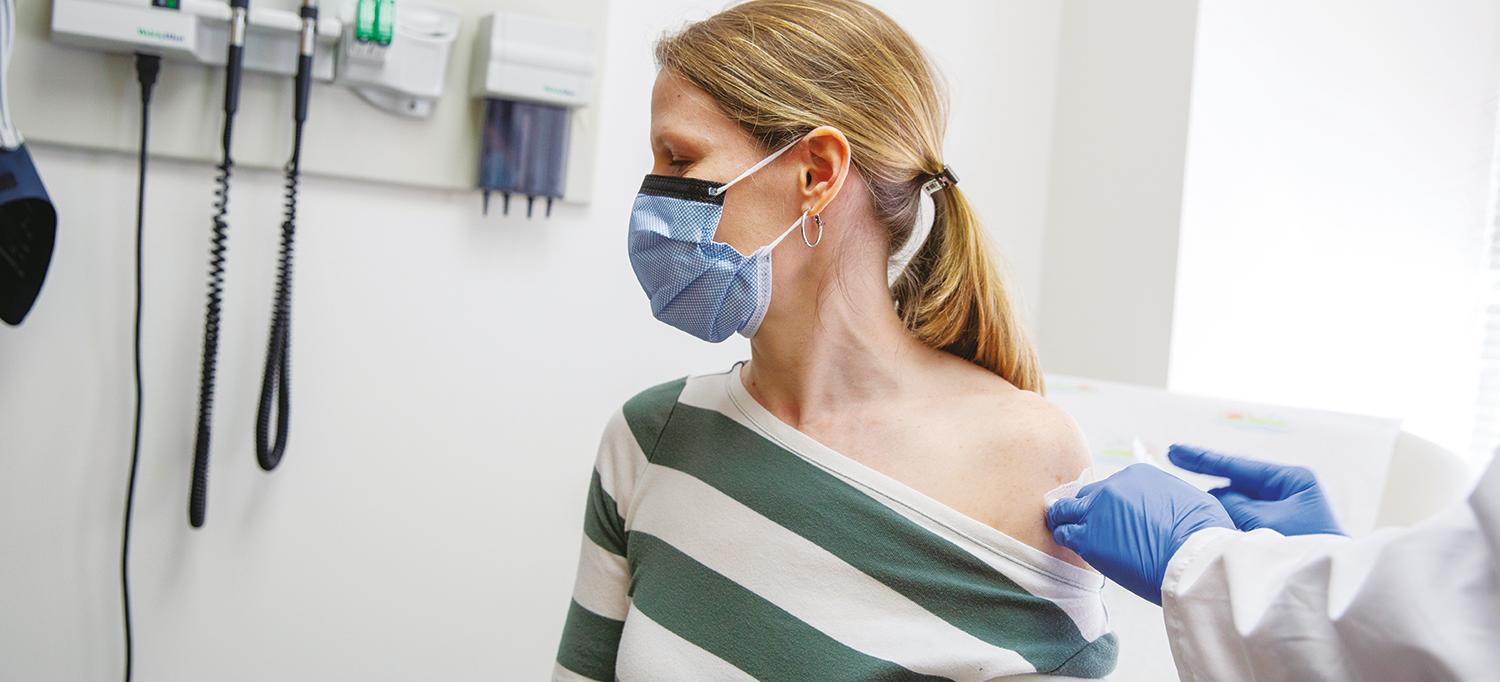
Patient Melissa Honkanen volunteers to participate in a COVID-19 vaccine trial at NYU Langone that is investigating four vaccine candidates. Based on the trial’s encourging results, the Vaccine Center has since helped launch a large international trial to test the efficacy of the top candidates.
Photo: NYU Langone Staff
In mid-March of 2020, the coronavirus disease (COVID-19) tsunami began washing over New York City with terrifying force. Even though the virus had already overwhelmed Wuhan, China, and walloped northern Italy and Spain, its speed and devastation took New Yorkers by surprise. “We knew very little about it, and it hit hard very suddenly, so it was really all hands on deck,” says Judith S. Hochman, MD, senior associate dean for clinical sciences and the Harold Snyder Family Professor of Cardiology at NYU Langone Health. Medical researchers and clinicians were simultaneously caring for desperately ill patients, scouring reports for new information, and rapidly assembling their own clinical trials to learn all they could about the virus and disease.
NYU Langone had persevered through Hurricane Sandy in 2012. But the slow-motion disaster of a poorly understood pandemic besieging New York has presented an entirely new challenge. “There is something very different about this, and that is the unknown,” says Dafna Bar-Sagi, PhD, vice dean for science and the Saul J. Farber Professor of Biochemistry and Molecular Pharmacology. “The circumstances are unprecedented.”
So, too, has been the response. By the end of June, NYU Langone had launched or joined 14 trials investigating a range of potential therapies. The health system’s hospitals alone had evaluated over 25,000 patients for COVID-19—a number that has continued to climb. A shared sense of responsibility and teamwork have helped NYU Langone achieve an astonishing output of virus-related research findings.
Among the most noteworthy advances emanate from NYU Langone’s Vaccine Center, directed by Mark J. Mulligan, MD, the Thomas S. Murphy Sr. Professor of Medicine and director of the Division of Infectious Diseases and Immunology. In April, the National Institutes of Health selected the Vaccine Center to be among 10 vaccine trial and evaluation units, and Dr. Mulligan and colleagues helped launch a major clinical trial of a vaccine candidate developed by the pharmaceutical company AstraZeneca. In a separate collaboration, the Vaccine Center has tested 4 vaccine candidates made by Pfizer Inc. and BioNTech, and the successful trial paved the way for a massive international trial of 30,000 volunteers.
“We’re making efforts to enroll people with higher than average risk for COVID-19 infection—racial and ethnic minorities, frontline workers, first responders, and essential workers,” Dr. Mulligan says. “It’s a privilege to be part of what will hopefully be a solution to this horrible pandemic.”
Another positive step involves a treatment: NYU Langone researchers have launched a trial testing an experimental drug called remdesivir and found that hospitalized patients who receive it recover faster than those who do not. Based on the data, the U.S. Food and Drug Administration (FDA) gave remdesivir an emergency-use authorization as a COVID-19 treatment.
Kerry L. Dierberg, MD, MPH, hospital epidemiologist and infectious diseases section chief at NYC Health + Hospitals/Bellevue, leads the remdesivir trial at NYU Langone and Bellevue Hospital, which are among the 68 international sites that together enroll more than 1,000 patients. “For me, it is pretty remarkable to see how quickly people have mobilized to facilitate research amid an outbreak,” she says. The crush of new cases, lack of existing therapies, and surprising revelations about how the disease spreads and attacks patients add to the extreme research challenge. Amy V. Rapkiewicz, MD, associate professor of pathology at NYU Langone Hospital—Long Island, reported a particularly sobering moment in the midst of performing her third COVID-19–related autopsy. That thorough postmortem examination, on April 3, revealed blood clots throughout the patient’s body. “Every single organ was filled with clots,” she says. “It was really startling because I just didn’t expect that degree of involvement.”
Dr. Rapkiewicz published a study that suggests COVID-19 cases are often hard to manage because the damage can extend well beyond the lungs and include features like unusual microclots in the heart and other organs. “Doing the autopsies and seeing the different pathologies in these organs will help in defining how we look at this disease and how we treat it,” Dr. Rapkiewicz says.
As cases have mounted, other surprises have emerged. Jennifer L. Lighter, MD, an epidemiologist at Hassenfeld Children’s Hospital at NYU Langone, says researchers in China had cited older age, diabetes, and cardiovascular disease as the main risk factors for a more severe course of disease. She and colleagues, though, notice that many of NYU Langone’s younger COVID-19 patients requiring intubation are obese. The researchers’ retrospective study finds that for patients younger than 60, obesity is a significant risk factor—a finding that is now included in the Center for Disease Control and Prevention’s list of risk factors.
Like other investigators, Dr. Lighter juggles multiple responsibilities, such as keeping the health system up-to-date on fast-changing clinical guidelines and clinical trial data. “Everybody pulls together and helps in every way they can,” Dr. Lighter says. “It is really beautiful to witness and be a part of.”
Researchers and clinicians work 24/7 to document new symptoms and complications, process tissue and blood samples, and develop new clinical trials. Investigators write protocols from scratch and obtain FDA permission to enroll patients. “We’ve never seen things move this fast,” Dr. Hochman says. “It is truly remarkable.”
The Office of Science and Research’s Clinical Research Support Unit and the Clinical and Translational Science Institute help with trial development, coordination, and training. The Division of Biostatistics aids in designing the studies and trials and in analyzing the resulting observational data, while the DataCore platform and staff, based in the information technology department, allow researchers to build new databases for their clinical studies and to gather, store, understand, and share a huge volume of information.
Staff who had been working on non–COVID-19–related trials train and redeploy to assist with the new efforts. NYU Langone’s science review committees and institutional review boards, tasked with assessing and approving the scientific basis and safety of the new clinical trials, meet on nights and weekends to accommodate all of the emergency requests.
Medical ethicists help the researchers think about how to prioritize the trials and expand access to care. With overlapping eligibility criteria, investigators have to decide which patients might be best suited for which trials, and how to divide the hospital floors accordingly. “We realize that there needs to be a seamless coordination between the people caring for the patients and the people designing the clinical trials,” Dr. Bar-Sagi says.
At the height of the spring surge, nearly every floor of Kimmel Pavilion was reconfigured into a COVID-19–specific intensive care unit. A meticulous attention to detail keeps NYU Langone from running out of beds and allows healthcare workers and staff to focus on safely delivering patient care and improving care through clinical research.
Beyond the heroic individual efforts, Dr. Hochman and Dr. Bar-Sagi attribute the success to deep investments in research infrastructure. “We are critically dependent on the infrastructure to pull all of this together,” Dr. Hochman says. “Every element has contributed.”
On March 28, 2020, NYU Langone’s Center for Biospecimen Research and Development began collecting and banking viral, blood, DNA, and tissue samples from hospitalized patients. Iman Osman, MD, the Rudolf L. Baer Professor of Dermatology and associate dean for translational research support, says the extraordinary effort must prioritize the care of patients who are seriously ill. “The other challenge to adjust to is the unprecedented volume,” Dr. Osman says, citing the need to quickly scale up to handle hundreds of samples per day. By the first week of July, the center had collected nearly 40,000 samples from almost 6,500 patients.
“We immediately distribute specimens for research that could feed directly into clinical care,” Dr. Osman says. Some go to help improve and validate clinical tests. Other samples go to the Genome Technology Center, where 9 researchers sequenced the viral RNA from more than 1,000 patients in 2 months. “We have turned around on a dime and taken existing infrastructure, such as the sequencers and the robots in my lab, to work exclusively on the COVID-19 samples,” says Adriana Heguy, PhD, professor of pathology and director of the Genome Technology Center.
Dr. Heguy recalls watching in horror as the coronavirus swept through Italy’s Lombardy region, where some of her relatives live. As cases began appearing in New York, her own research confirmed her fears that authorities were paying too much attention to travel from China and not enough to the many flights still arriving from Europe. “My thought was that I was watching a train wreck that was coming toward us,” she says.
Around the world, scientists have sequenced the SARS-CoV-2 genome from patients and added it to a central database. Based on new mutations as the virus spread, researchers can compare similarities among outbreaks in successive locations. The result is something like a family tree that shows the relationship among viral isolates around the world, allowing researchers to see how COVID-19 is spreading. “It’s like making a subway map,” Dr. Heguy says.
She and colleagues added more than 860 SARS-CoV-2 sequences to the international database. Their analysis, conducted in collaboration with Matthew T. Maurano, PhD, assistant professor of pathology, and Matija Snuderl, MD, associate professor of pathology, suggests that the virus has been circulating in New York since at least mid-February of 2020—much earlier than initially thought. Their data show that most of the circulating coronavirus came from Europe, not from China, and reveals at least 88 independent chains of transmission in New York.
NYU Langone’s secure Biosafety Level 3 Lab, a federally regulated facility for studying potentially lethal microbes that spread through inhalation, likewise plays a critical role. Virologists and microbiologists partner with Dr. Mulligan and other researchers to accommodate the new COVID-19 studies, allowing the collaborators to investigate whether the coronavirus can enter specific cells in the body and how it reacts to antibodies and experimental drugs. The valuable research findings are a testament to the early and sustained investment in infrastructure “before we recognized that it would be so needed,” Dr. Bar-Sagi says. “It is incredible to watch it being used for the very purpose that it was built for.”
Dr. Hochman says the huge effort will leave NYU Langone far better prepared for the next COVID-19 surge or pandemic. The experience, she says, also underscores the importance of worldwide collaboration. Completing clinical trials and securing badly needed answers may require close coordination among multiple centers in different countries. “We have the advantage of having a superb track record in leading multi-institutional trials, so people ask us to lead some of these collaborations,” Dr. Bar-Sagi adds. “It’s going to take many people working together to get it done.”