A Newly Refined Surgical Tool Pioneered by Dr. Larry Chinitz, Director of the Heart Rhythm Center, Promises to Enhance Patient Safety & Improve Outcomes
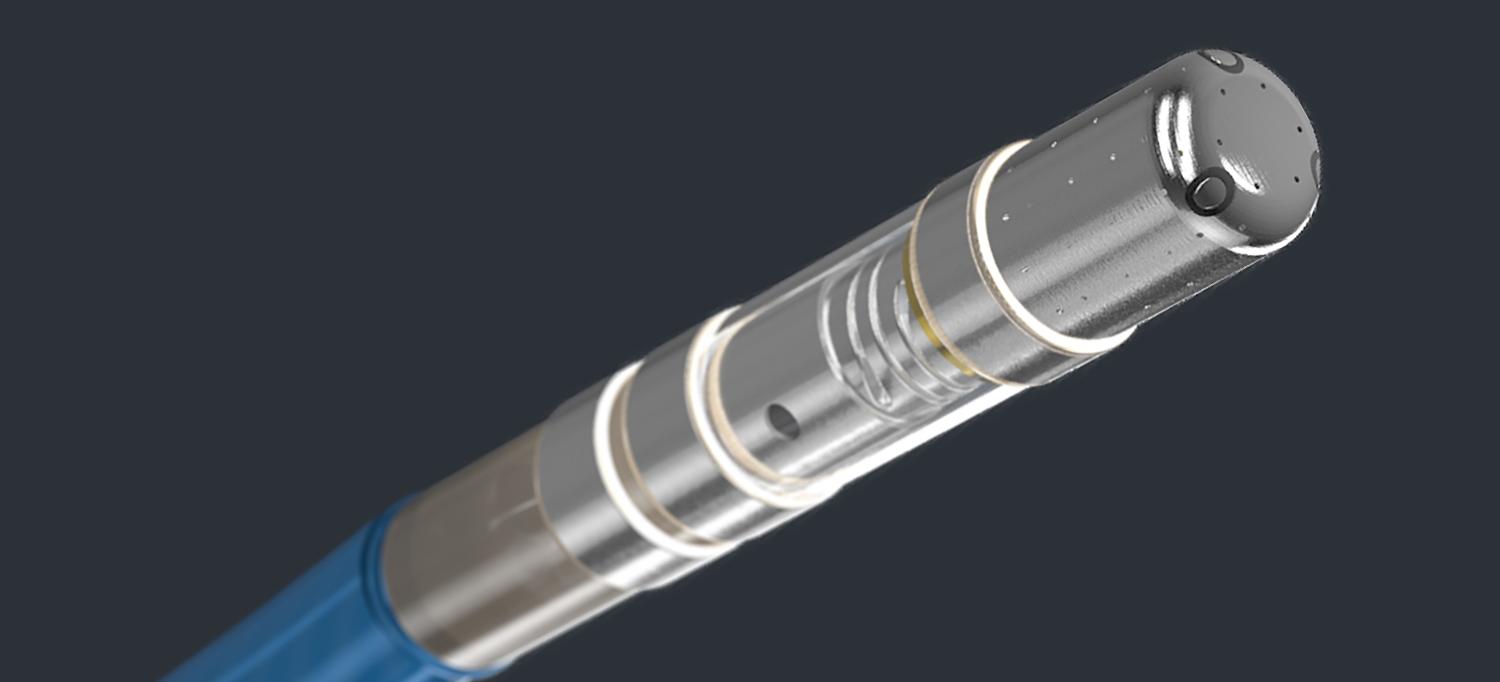
The Qdot Micro generates roughly three times the heating power of conventional ablation devices. “It can potentially cut the procedure length in half,” notes Dr. Chinitz, the Alvin Benjamin and Kenneth Coyle Sr., Family Professor of Medicine and Cardiac Electrophysiology.
Photo courtesy of Biosense Webster
It may not look like much—just a tiny metal tip attached to a thin tube—but this device could prove to be a game changer for some of the roughly 4 million people in the United States who have an irregular heartbeat, or arrhythmia. This unsettling condition results when electrical signals in the heart short-circuit, causing the heart to beat too quickly, too slowly, or irregularly. Symptoms include dizziness, chest pain, shortness of breath, and in extreme cases, sudden cardiac arrest.
Many patients find relief through a combination of blood pressure medications and lifestyle changes that eliminate stimulants such as nicotine and caffeine. But when symptoms persist, cardiologists often recommend a nonsurgical procedure called catheter ablation to destroy, or ablate, the misfiring heart cells. While the procedure can restore a normal heart rhythm, it can be taxing. Over the course of two to four hours, depending on the case, a team of doctors, guided by X-ray imaging, delicately thread a thin catheter through a blood vessel in the groin and up into the heart. Once it is properly positioned, they use the tip of the device to burn misfiring cardiac cells.
Now, a new advance in the field could make the procedure easier on patients. In February, Larry A. Chinitz, MD, director of NYU Langone’s Heart Rhythm Center, became the first physician in the United States to trial a new ablation device, called the Qdot Micro, that generates roughly three times the heating power of conventional devices. “It can burn away faulty cells in as little as four seconds and potentially cut the procedure length in half,” notes Dr. Chinitz, the Alvin Benjamin and Kenneth Coyle, Sr., Family Professor of Medicine and Cardiac Electrophysiology. For patients, this means less sedation and reduced exposure time to X-rays. The shorter ablation time also reduces the risk of damaging healthy surrounding tissue.
The landmark trial is part of an investigational study underway at 30 sites nationwide involving 185 patients with atrial fibrillation, the most common form of arrhythmia. It’s no surprise that Dr. Chinitz is taking the lead in the trial. A pioneer of catheter ablation and other novel treatments for heart arrhythmia, he heads one of the busiest arrhythmia centers in the country. His team is now treating two or three patients per month with the new device, and early results are encouraging. “This is a significant new technology,” he says, “and we expect it will improve both patient safety and clinical outcomes.”

