Diane M. Simeone, MD
PHOTO: JONATHAN KOZOWYK
From revealing genetic underpinnings to testing new clinical protocols, Perlmutter Cancer Center research teams are targeting pancreatic cancers in innovative ways.
New Pancreatic Cancer Center Focuses on Research, Early Detection
Building on its robust research and strong clinical framework, Perlmutter Cancer Center launched its new, multidisciplinary Pancreatic Cancer Center in July 2017. Renowned pancreatic cancer surgeon and researcher Diane M. Simeone, MD, the Laura and Isaac Perlmutter Professor of Surgery, professor of pathology, and associate director of translational research, directs this combined clinical and research effort, leveraging insights and expertise from across the institution.
The center will tackle long-standing questions about pancreatic cancer’s diagnosis, treatment, and prevention, and further expand upon recent research findings described below.
Perlmutter Cancer Center is 1 of 12 national sites participating in the Pancreatic Cancer Action Network’s Precision Promise, a large-scale precision medicine trial led by Diane M. Simeone, MD.
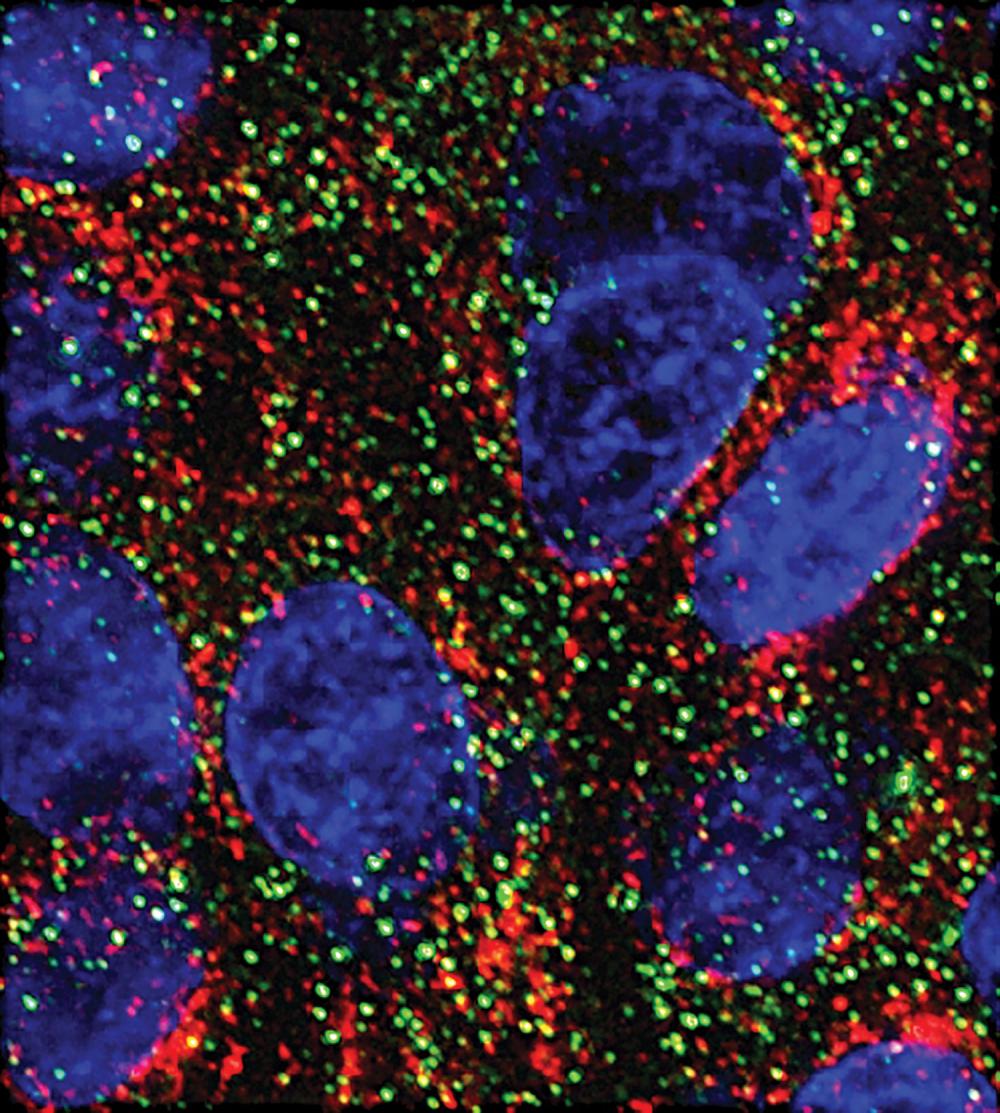
Stress Granules Protect Pancreatic Cancer Cells
Pancreatic cancer cells protect themselves by producing stress granules that lessen the effects of chemotherapy, according to research from the laboratory of Perlmutter Cancer Center investigator Dafna Bar-Sagi, PhD, professor of biochemistry and molecular pharmacology and medicine, senior vice president and vice dean for science, and chief scientific officer. Dr. Bar-Sagi and collaborators reported in the December 2016 issue of Cell that cancer cells with mutations in the KRAS gene make six times more stress granules than cells without the mutations when exposed to radiation or the chemotherapy agent oxaliplatin. The team also produced the first-ever images of stress granules inside human pancreatic tumors.
“Our results explain why KRAS mutant cells are so good at resisting treatment and suggest a way to make them many times more vulnerable to existing chemotherapies,” says Dr. Bar-Sagi. “Given the lack of good treatments for these patients, the ability to interfere with this coping mechanism would be revolutionary.”
Immune Action Revealed in Existing Therapy
Researchers have revealed a second, previously unappreciated mechanism of action for a decades-old cancer drug. Initially designed to prevent cancer cells from multiplying, nab-paclitaxel (Abraxane®) can also stimulate the immune system to attack pancreatic tumors, according to research in Dr. Bar-Sagi’s laboratory.
By studying a mouse model of pancreatic cancer and laboratory-grown macrophage cells, the research team showed that the drug causes macrophages in the tumor microenvironment to transition to an immune-activating state. The team reported their findings in February 2017 in Cancer Immunology Research.
“Our study reveals a previously unappreciated role for Abraxane® in tumor immunology,” says Dr. Bar-Sagi. “In doing so, it suggests ways to improve the drug and argues for its inclusion in new kinds of combination treatments.”
Severing Pancreatic Cancer’s Fuel Lines
Pancreatic cancers are known to have adaptive metabolic networks that sustain their proliferation, and exploiting this difference from normal cells could reveal new therapeutic targets, according to a collaborative study led by Alec Kimmelman, MD, PhD, professor of radiation oncology, the Anita Steckler and Joseph Steckler Chair of Radiation Oncology, and co-leader of the Cancer Cell Biology Research Program.
The researchers, whose results were published in July 2017 in Nature Communications, found that simply shutting down a novel metabolic pathway they had previously identified causes pancreatic ductal adenocarcinoma (PDAC) cells to shift their metabolic networks to sustain themselves with available nutrients. Building on this discovery, the team conducted a series of proteomic and metabolomic analyses to identify promising combination therapies that can target more than one metabolic pathway.
“This work highlights how metabolically adaptive pancreatic cancers are, offering us the opportunity to understand these adaptations and hopefully develop effective therapeutic combinations,” says Dr. Kimmelman.
Researchers Selected to Co-Lead and Collaborate in Pancreatic Cancer Translational Research Team
Nine pancreatic cancer researchers from Perlmutter Cancer Center have been selected to lead and collaborate in a Cancer Interception Translational Research Team by Stand Up To Cancer (SU2C) and the Lustgarten Foundation for Pancreatic Cancer Research.
The team, co-led by Dr. Kimmelman and David P. Ryan, MD, of Massachusetts General Hospital, was awarded $2.6 million to develop novel approaches to treat and evaluate early pancreatic cancer. In particular, they will assess the benefits of adding certain drugs to chemotherapy treatments in the hopes of minimizing disease recurrence.
“Not only do we believe that this clinical trial has the potential to change the standard of care of how we approach this deadly disease, the analysis of clinical specimens collected from the initiative will be quite powerful in informing future trials and further improving patient outcomes,” says Dr. Kimmelman. “For example, our group will look at how tumor cell metabolism is impacted by treatment using our newly created metabolomics core facility, led by Drew Jones, PhD, and Michael E. Pacold, MD, PhD.”
In addition to Dr. Kimmelman, the Perlmutter Cancer Center team includes:
- Dafna Bar-Sagi, PhD
- Pratip Chattopadhyay, PhD, associate professor of pathology and director of the Precision Immunology Laboratory in the Division of Advanced Research Technologies
- Deirdre J. Cohen, MD, assistant professor of medicine
- Kevin L. Du, MD, PhD, assistant professor of radiation oncology and director of the Radiation Oncology Residency Program
- Michael E. Pacold, MD, PhD, assistant professor of radiation oncology and chair of the faculty advisory committee for the Metabolomics Core Resource Laboratory
- Diane M. Simeone, MD
- Kwok-Kin Wong, MD, PhD, the Anne Murnick Cogan and David H. Cogan Professor of Oncology and director of the Division of Hematology and Medical Oncology
“The selection of our group as a Cancer Interception Translational Research Team by SU2C and the Lustgarten Foundation is a tremendous honor. It underscores our commitment to push the boundaries of pancreatic cancer research so that we can provide patients with this relentless disease access to the most innovative treatments and world-class care.” —Dafna Bar-Sagi, PhD
These articles also appear in the Gastroenterology & GI Surgery 2017 Year in Review.