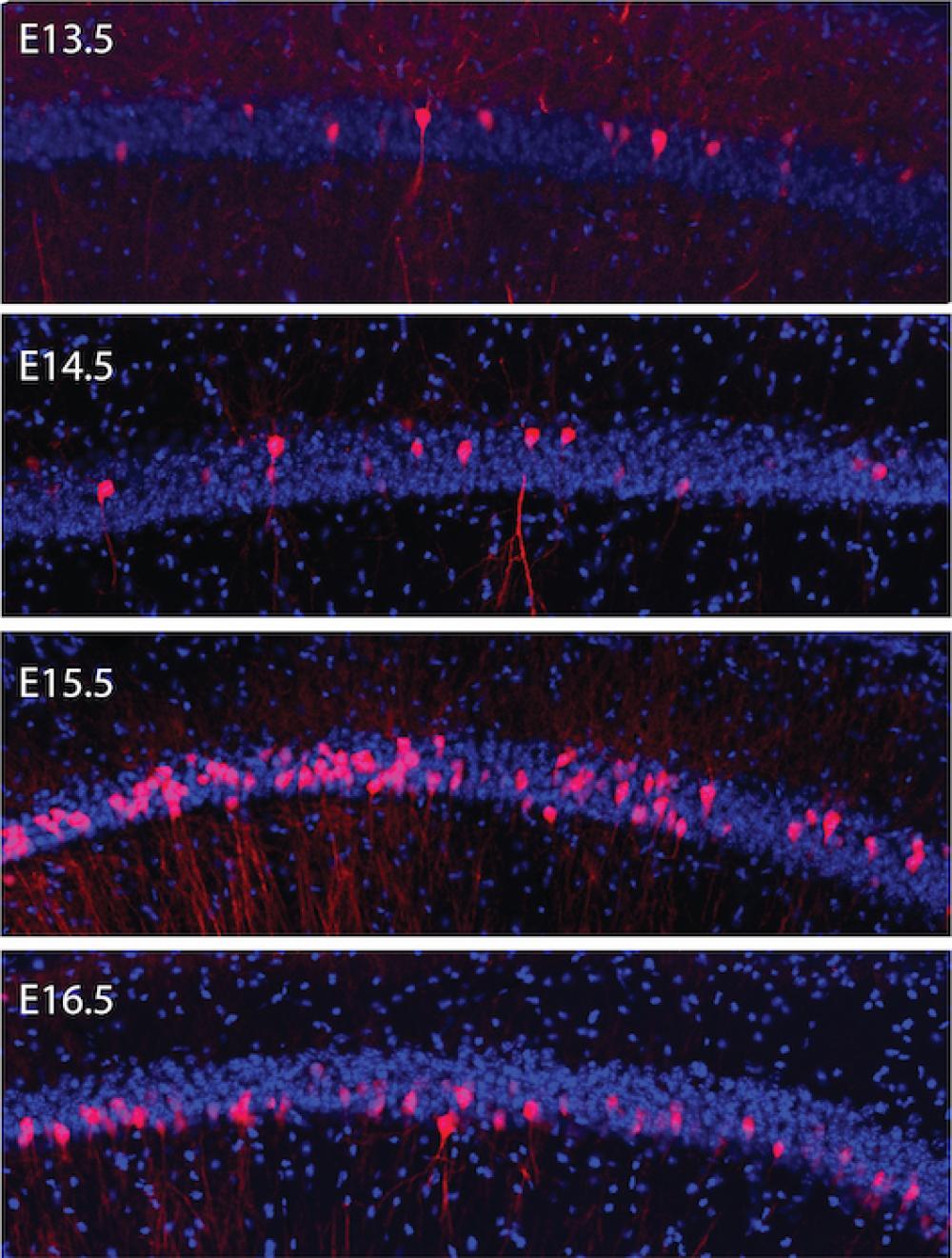
Brain cells with the same “birthdate” are more likely to wire together into cooperative signaling circuits that carry out many functions, including the storage of memories, a new study finds.
Led by researchers from NYU Grossman School of Medicine, the new study on the brains of mice developing in the womb found that brain cells (neurons) with the same birthdate showed distinct connectivity and activity throughout the animals’ adult lives, whether they were asleep or awake.
Published online August 22 in Nature Neuroscience, the findings suggest that evolution took advantage of the orderly birth of neurons—by gestational day—to form localized microcircuits in the hippocampus, the brain region that forms memories. Rather than attempting to create each new memory from scratch, the researchers suggest, the brain may exploit the stepwise formation of neuronal layers to establish neural templates, like “Lego pieces,” that match each new experience to an existing template as it is remembered.
These rules of circuit assembly would suggest that cells born together are more likely to encode memories together, and to fail together, potentially implicating neuronal birthdate in diseases like autism and Alzheimer’s disease, say the authors. With changes to the number of cells born at different days, the developing brain may be vulnerable on some gestational days to viral infections, toxins, or alcohol.
“Our study’s results suggest that which day a hippocampal neuron is born strongly influences both how that single cell performs, and how populations of such cells signal together throughout life,” says senior study author György Buzsáki, MD, PhD, the Biggs Professor of Neuroscience in the Department of Neuroscience and Physiology at NYU Langone Health. “This work may reshape how we study neurodevelopmental disorders, which have traditionally been looked at through a molecular or genetic, rather than a developmental, lens,” says Dr. Buzsáki, also a member of the Neuroscience Institute at NYU Langone.
New Understanding of Memory Storage
The current study’s innovation rests on tracking the activity of neurons of a given birthdate into adulthood. To accomplish this, the researchers relied on a technique that allowed them to transfer DNA into cells that were undergoing division into neurons in the womb. The DNA expressed markers that tagged brain cells that were born on same day, akin to a barcode. This labeling method then enabled the researchers to study these neurons in the adult animal.
Using a combination of techniques, the new study found that neurons of the same birthdate tend to “co-fire” together, characterized by synchronized swings in their positive and negative charges, allowing them to transmit electrical signals collectively. A likely reason for the co-firing, say the authors, is that neurons with the same birthdate are connected via shared neurons.
Past work had shown that activity in the hippocampus can be described in terms patterns of collective neuronal activity during waking and sleep. During sleep, for instance, when each day’s memories are consolidated for long-term memory storage, hippocampal neurons engage in a cyclical burst of activity called the “sharp wave-ripple,” named for the shape it takes when captured graphically by EEG, a technology that records brain activity with electrodes.
“Our results show that neurons born on the same day become part of the same cooperating assemblies, and participate in the same sharp wave-ripples and represent the same memories,” says first author Roman Huszár, a graduate student in Dr. Buzsáki’s lab. “These relationships, and the pre-set templates they encode, have a key implication for hippocampal function: the storage of a memory about a place or event.”
Moving forward, the team plans additional experiments to identify the genes active in the same birthdate neurons in different brain regions, and to test their role in memory formation and behavior.
Along with Dr. Buzsáki and Huszár, the other study authors were Yunchang Zhang from the Neuroscience Institute at NYU Langone and the Center for Neural Science at NYU; and Heike Blockus of the Department of Neuroscience and the Zuckerman Mind Brain Behavior Institute at Columbia University. Funding for the study was provide by National Institutes of Health grants RO1 MH122391 and U19 NS107616.
Media Inquiries
Greg Williams
Phone: 212-404-3500
gregory.williams@nyulangone.org